Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, Setting, and Nutrition Policy
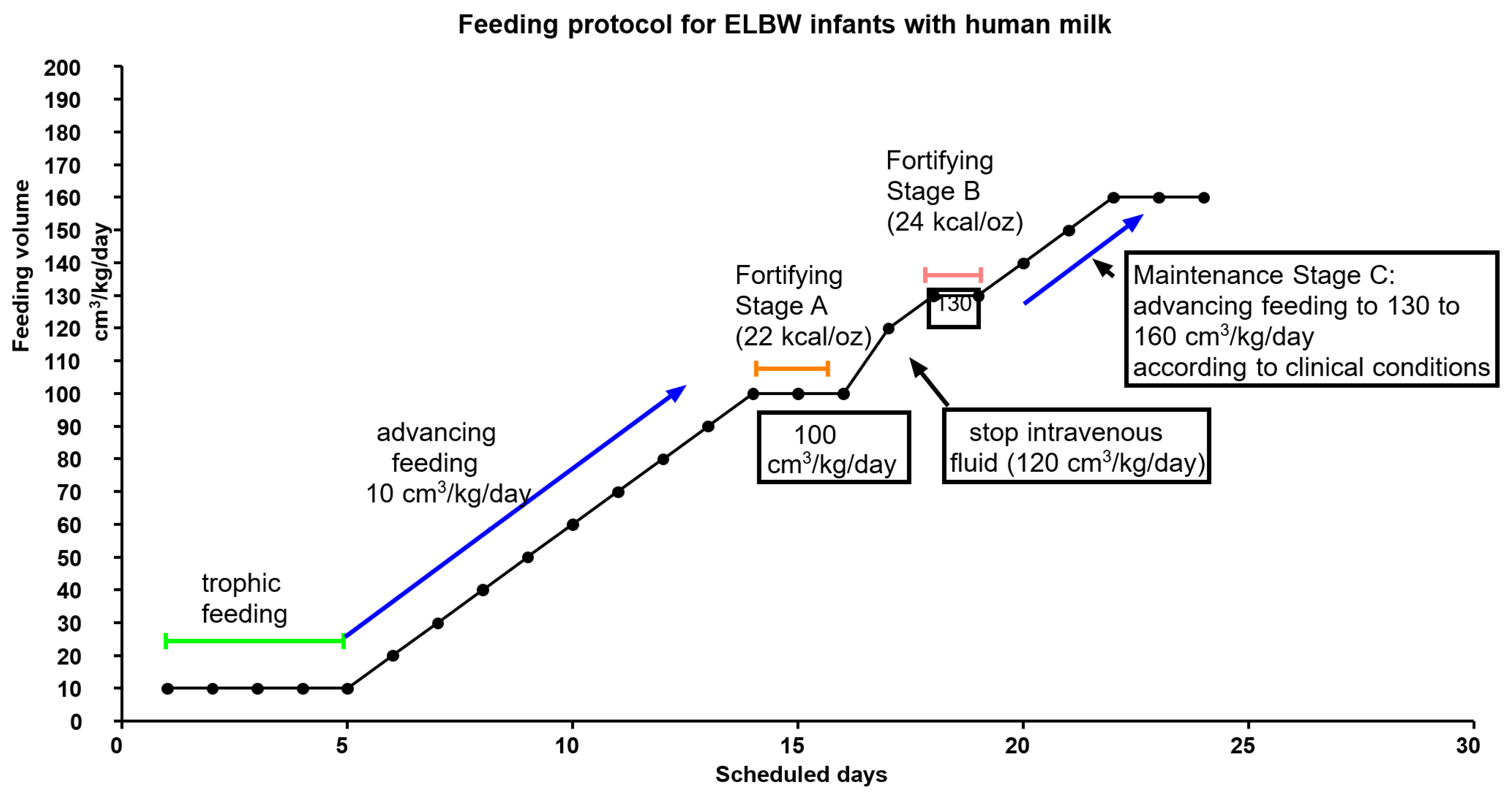
2.1.1. Nutrition Policy in Hospitalization
2.1.2. Nutrition Policy in the Pre-Discharge Period until 24 months Old
2.2. QI Project
2.3. Follow-Up Program of NICU Graduates
2.4. Study Design
2.5. The Statistics and Analysis
3. Results
4. Discussion
4.1. Overall Nutrition Management in Extremely Preterm Infants
4.2. Feeding and Fortification in Extremely Preterm Infants
4.3. The Problem of Powder Form Fortification
4.4. The Advangtage and Disadvantage of the Alternatives of Liquid Fortifier
4.5. Additional Benifits of Maintaining HM Use in Preterm Infants
4.6. Limitations and Strengths of This Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- de Halleux, V.; Pieltain, C.; Senterre, T.; Rigo, J. Use of donor milk in the neonatal intensive care unit. Semin. Fetal Neonatal. Med. 2017, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tudehope, D.I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 2013, 162, S17–S25. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 6, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, Y.; Ma, L.Y.; Lin, H.C. The role of immunonutrients in the prevention of necrotizing enterocolitis in preterm very low birth weight infants. Nutrients 2015, 7, 7256–7270. [Google Scholar] [CrossRef] [PubMed]
- Buckle, A.; Taylor, C. Cost and cost-effectiveness of donor human milk to prevent necrotizing enterocolitis: Systematic review. Breastfeed. Med. 2017, 12, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. 2018, 38, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor human milk protects against bronchopulmonary dysplasia: A systematic review and meta-analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanescu, B.M.; Gillam-Krakauer, M.; Stefanescu, A.R.; Markham, M.; Kosinski, J.L. Very low birth weight infant care: Adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum. Dev. 2016, 94, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Cole, T.J.; Lister, G.; Leeson-Payne, C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet 1992, 339, 261–264. [Google Scholar] [CrossRef]
- Brune, K.D.; Donn, S.M. Enteral feeding of the preterm infant. NeoReviews 2018, 19, e645–e653. [Google Scholar] [CrossRef]
- Hay, W.W.; Ziegler, E.E. Growth failure among preterm infants due to insufficient protein is not innocuous and must be prevented. J. Perinatol. 2016, 36, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Boquien, C.-Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of human milk for preterm infants: Update and recommendations of the European Milk Bank Association (EMBA) Working Group on human milk fortification. Front. Pediatrics 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Steele, C. Best practices for handling and administration of expressed human milk and donor human milk for hospitalized preterm infants. Front. Nutr. 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorling, J.; Abbott, J.; Berrington, J.; Bosiak, B.; Bowler, U.; Boyle, E.; Embleton, N.; Hewer, O.; Johnson, S.; Juszczak, E.; et al. Controlled trial of two incremental milk-feeding rates in preterm infants. N. Engl. J. Med. 2019, 381, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Piemontese, P.; Liotto, N.; Menis, C.; Mallardi, D.; Tabasso, C.; Perrone, M.; Bezze, E.; Plevani, L.; Roggero, P.; Mosca, F. Effect of target fortification on osmolality and microbiological safety of human milk over time. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 381–385. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawoger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e1. [Google Scholar] [CrossRef] [Green Version]
- Neofax 2011, 24th ed.; Thomson Reuters: Montvale, NJ, USA, 2011.
- Pillai, A.; Albersheim, S.; Matheson, J.; Lalari, V.; Wei, S.; Innis, S.M.; Elango, R. Evaluation of a concentrated preterm formula as a liquid human milk fortifier in preterm babies at increased risk of feed intolerance. Nutrients 2018, 10, 1433. [Google Scholar] [CrossRef] [Green Version]
- Taiwan Society of Neonatology. Recommendation on Nutritional Care of Taiwan Preterm Infants; Taiwan Society of Neonatology: Taichung City, Taiwan, 2015. [Google Scholar]
- Su, B.H. Optimizing nutrition in preterm infants. Pediatr. Neonatol. 2014, 55, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Health Promotion Administration, Ministry of Health and Welfare. Children’s Health Booklet; Health Promotion Administration Ministry of Health and Welfare: Taipei, Taiwan, 2017.
- American Academy of Pediatrics. Caring for Your Baby and Young Child: Birth to Age 5, 7th ed.; Bantam: New York, NY, USA, 2019. [Google Scholar]
- Lin, Y.C.; Lin, Y.J.; Lin, C.H. Growth and neurodevelopmental outcomes of extremely low birth weight infants: A single center’s experience. Pediatr. Neonatol. 2011, 52, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Abbott Nutrition. Pediatric Nutrition Product Guide 2016; Abbott Laboratories: Columbus, OH, USA, 2016; Volume 2020. [Google Scholar]
- Wang, L.W.; Lin, Y.C.; Tu, Y.F.; Wang, S.T.; Huang, C.C.; Taiwan Premature Infant Developmental Collaborative Study Group. Isolated cystic periventricular leukomalacia differs from cystic periventricular leukomalacia with intraventricular hemorrhage in prevalence, risk factors and outcomes in preterm infants. Neonatology 2017, 111, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Lin, Y.C.; Wang, S.T.; Huang, C.C.; Taiwan Premature Infant Developmental Collaborative Study Group. Identifying risk factors shared by bronchopulmonary dysplasia, severe retinopathy, and cystic periventricular leukomalacia in very preterm infants for targeted intervention. Neonatology 2018, 114, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Younge, N.; Goldstein, R.F.; Bann, C.M.; Hintz, S.R.; Patel, R.M.; Smith, P.B.; Bell, E.F.; Rysavy, M.A.; Duncan, A.F.; Vohr, B.R.; et al. Survival and neurodevelopmental outcomes among periviable infants. N. Engl. J. Med. 2017, 376, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.G.; Baer, R.J.; Partridge, J.C.; Kuppermann, M.; Franck, L.S.; Rand, L.; Jelliffe-Pawlowski, L.L.; Rogers, E.E. Survival and major morbidity of extremely preterm infants: A population-based study. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K.; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, E.E. Meeting the nutritional needs of the low-birth-weight infant. Ann. Nutr. Metab. 2011, 58 (Suppl. 1), 8–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, F.; Hu, X.; Cao, Y.; Latour, J.M. Oropharyngeal colostrum administration in very low birth weight infants: A randomized controlled trial. Pediatr. Crit. Care Med. 2017, 18, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Knafl, G.; Thoyre, S.; Brandon, D. Factors associated with feeding progression in extremely preterm infants. Nurs. Res. 2015, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Jadcherla, S.R.; Wang, M.; Vijayapal, A.S.; Leuthner, S.R. Impact of prematurity and co-morbidities on feeding milestones in neonates: A retrospective study. J. Perinatol. 2010, 30, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, A.A.; Li, P.; Parks, K.; Lal, C.V.; Martin, C.R.; Carlo, W.A. Early progressive feeding in extremely preterm infants: A randomized trial. Am. J. Clin. Nutr. 2018, 107, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Tsai, M.L.; Chen, C.C.; Lin, H.C. Early optimal nutrition improves neurodevelopmental outcomes for very preterm infants. Nutr. Rev. 2014, 72, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Cheng, S.W.; Wu, T.Z.; Fang, L.J. Characteristics of the first human milk bank in Taiwan. Pediatr. Neonatol. 2013, 54, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modanlou, H.D.; Lim, M.O.; Hansen, J.W.; Sickles, V. Growth, biochemical status, and mineral metabolism in very-low-birth-weight infants receiving fortified preterm human milk. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; McClead, R.E. Human milk fortifiers. J. Pediatr. Perinat. Nutr. 1987, 1, 65–74. [Google Scholar]
- Kemp, J.E.; Wenhold, F.A.M. Human milk fortification strategies for improved in-hospital growth of preterm infants. S. Afr. J. Clin. Nutr. 2016, 29, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Preparation of Small Volumes (10mL) Standard Strength Fortified M/DEBM. Available online: http://swmnodn.org.uk/wp-content/uploads/2020/05/additon-of-HMF-less-than-50ml-EBM-1.pdf (accessed on 18 July 2020).
- Willeitner, A.; Anderson, M.; Lewis, J. Highly concentrated preterm formula as an alternative to powdered human milk fortifier: A randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 574–578. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Mueller, E.; Blaser, M. Breast milk, formula, the microbiome and overweight. Nat. Rev. Endocrinol. 2018, 14, 510–511. [Google Scholar] [CrossRef]
- Guaraldi, F.; Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]

| Protocol ID | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Type | Powder HMF | Powder HMF | CPF | CPF |
| Dilution/mixing | 2 packets to 100 cm3 HM | 4 packets to 100 cm3 HM | SSC30 with HM, 1:2 volume ratio | SSC30 with HM, 1:1 volume ratio |
| Per 100 cm3 of mixed milk | ||||
| Energy, kcal | 73.1 | 79 | 79 | 84.3 |
| Protein, g | 1.84 | 2.34 | 2 | 2.23 |
| Iron, mg | 0.292 | 0.458 | 0.7 | 0.97 |
| Ca, mg | 82.2 | 138.1 | 7 | 103.7 |
| P, mg | 45.6 | 77.7 | 42 | 57.1 |
| Vitamin D, IU | 61 | 119 | 52 | 77 |
| Osmolality, mOsm/kg H2O | 297 | 329 | 302 | 310 |
| Place of mixture | bedsides | bedsides | central clean room | central clean room |
| Period-I | Period-II | p-Value | |
|---|---|---|---|
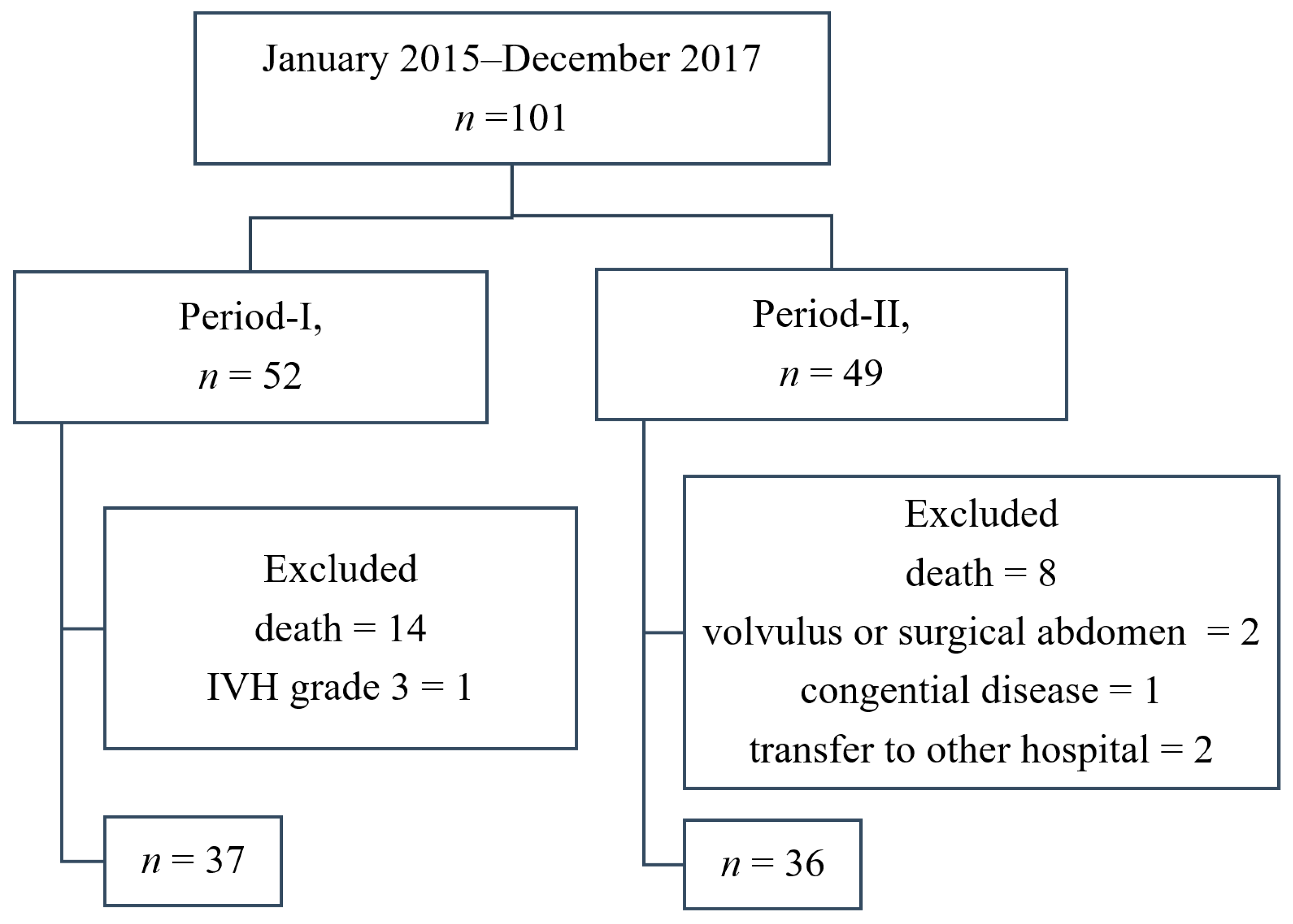
| Numbers of patients | 37 | 36 | |
| GA (mean ± SD), week (range) | 27.3 ± 2 (23.5–30.9) | 26.4 ± 2.1 (22.8–31.7) | 0.08 |
| BBW (mean ± SD), g (range) | 783.1 ± 131.2 (465-978) | 773.9 ± 143.7 (478-996) | 0.78 |
| BHC (mean ± SD), cm (range) | 23.5 ± 1.6 (18.5–25) | 23.1 ± 1.7 (19.5–25.5) | 0.32 |
| BBL (mean ± SD), cm (range) | 33.3 ± 2.4 (26–36) | 32.7 ± 2.9 (26.8–41) | 0.38 |
| Z-score of BBW | −0.81 ± 0.83 | −0.49 ± 0.75 | 0.09 |
| Z-score of BHC | −0.77 ± 0.8 | −0.81 ± 0.71 | 0.85 |
| Z-score of BBL | −0.82 ± 1.16 | −0.96 ± 2.64 | 0.77 |
| Male, n (% of total) | 20 (54) | 17 (47) | 0.20 |
| CS, n (% of total) | 10 (27) | 11 (35.6) | 0.80 |
| SGA, n (% of total) | 10 (27) | 5 (13.8) | 0.17 |
| Multiple pregnancy n (% of total) | 1 (2.7) | 2 (5.6) | 0.54 |
| Antenatal steroid, n (% of total) | 36 (97.3) | 36 (100) | 0.32 |
| APGAR scores | |||
| 1-min | 5.3 ± 1.7 | 4.3 ± 2.1 | 0.02 |
| 5-min | 7.5 ± 1.4 | 6.5 ± 2.2 | 0.02 |
| pH of 1st blood gas | 7.27 ± 0.07 | 7.26 ± 0.16 | 0.55 |
| Surfactant therapy, n (% of total) | 17 (45.9) | 17 (47.2) | 0.53 |
| HsPDA, n (% of total) | 13 (35.1) | 12 (33.3) | 0.57 |
| Early sepsis, n (% of total) | 4 (10.8) | 2 (5.5) | 0.67 |
| Period-I n = 37 | Period-II n = 36 | p-Value | |
|---|---|---|---|
| Postnatal age when nutrition management was commenced (mean ± SD), day | |||
| Central catheter insertions | 1.7 ± 1 | 1.7 ± 1.5 | 0.98 |
| Parenteral nutrition | 1 ± 0.1 | 1.1 ± 0.2 | 0.55 |
| Lipid infusion | 5.3 ± 4.1 | 3.9 ± 2 | 0.06 |
| First enteral feeding | 4 ± 1.6 | 4 ± 1.8 | 0.78 |
| Diet type before fortification | Exclusive BM or DBM | Exclusive BM or DBM | |
| When 1st fortification was commenced | |||
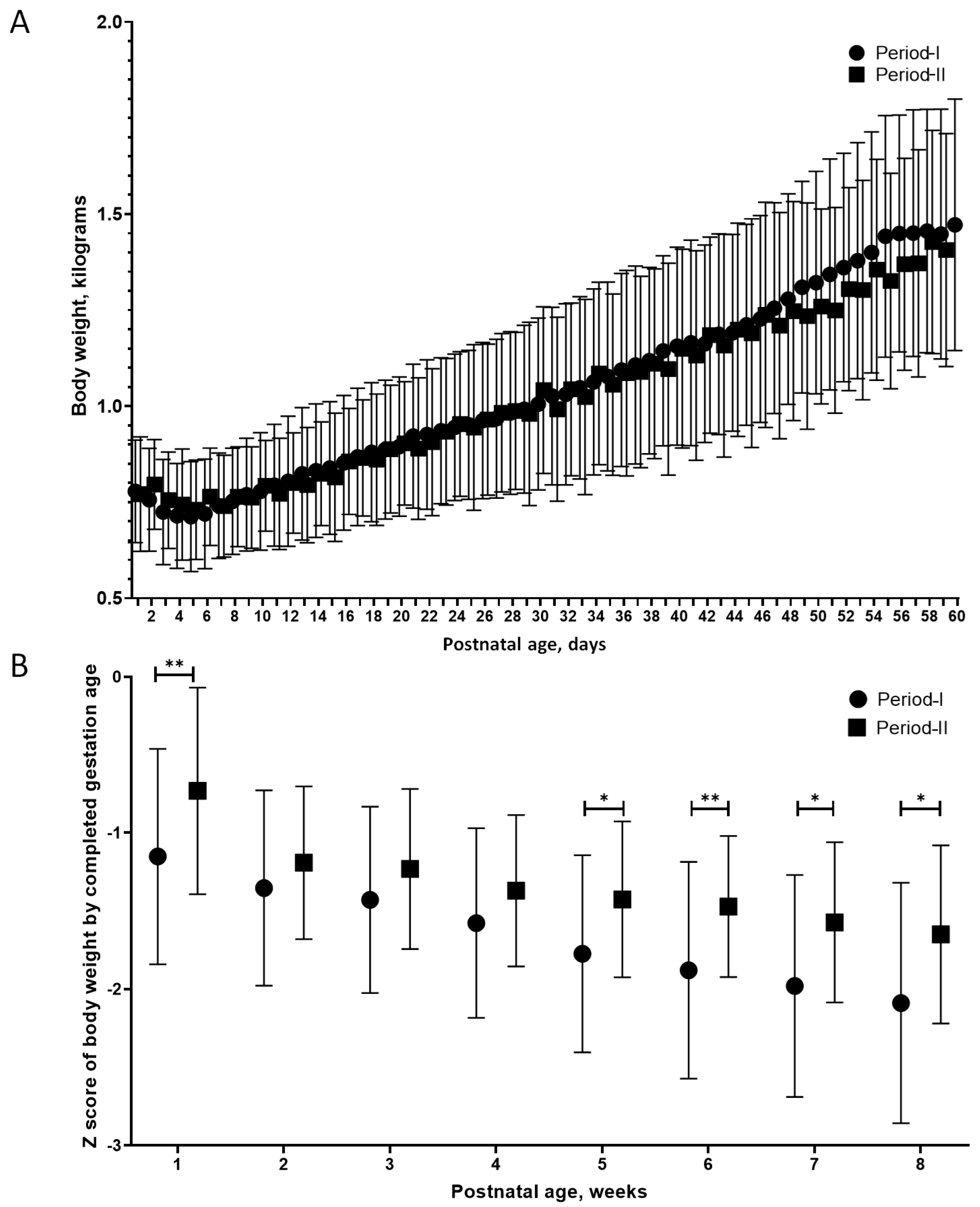
| Body weight (mean ± SD), g | 1051 ± 200 | 981 ± 232 | 0.18 |
| Postnatal age (mean ± SD), days | 34.2 ± 14.1 | 30.5 ± 16.5 | 0.3 |
| Postmenstrual age (mean ± SD), weeks | 32 ± 1.8 | 30.6 ± 2 | 0.002 |
| Days of the nutritional supplement before PMA 36 weeks (mean ± SD), day | |||
| NPO | 7.8 ± 7.6 | 7.8 ± 7.2 | 0.99 |
| Lipid infusion | 21 ± 10 | 25.8 ± 15.4 | 0.11 |
| Parenteral nutrition | 25.8 ± 12.2 | 30 ± 15.3 | 0.19 |
| Enteral feeding | 56.5 ± 13 | 61.9 ± 14.1 | 0.1 |
| Period-I | Period-II | |||
|---|---|---|---|---|
| p-value | ||||
| N | 37 | 36 | unadjusted | * adjusted |
| PMA at discharge (mean ± SD), weeks | 38.9 ± 2.6 | 39.17 ± 2.4 | 0.709 | |
| Length of hospital stay (mean ± SD), days | 82.4 ± 27.2 | 90 ± 28.5 | 0.247 | |
| Days on diuretics (mean ± SD), days | 23.3 ± 32.4 | 15.7 ± 22.8 | 0.035 | |
| Duration of mechanical ventilation (mean ± SD), days | 9 ± 15.4 | 8.3 ± 11.4 | 0.834 | |
| Postnatal steroid treatment, N (%) | 5 (13.5) | 10 (27.8) | 0.156 | |
| Neonatal morbidities during hospitalization | ||||
| CLD, N (%) | 11 (29.7) | 18 (50) | 0.097 | |
| LOS, N (%) | 9 (24.3) | 4 (11.1) | 0.221 | |
| Treated ROP, N (%) | 3 (8.1) | 4 (11.1) | 0.711 | |
| NEC ≥ stage 2, N (%) | 6 (16.2) | 4 (11.1) | 0.736 | |
| Metabolic bone disease, N (%) | 10 (27) | 8 (22.2) | 0.787 | |
| Anthropometry at term equivalent age (mean ± SD) | ||||
| PMA at evaluation, weeks | 38.5 ± 1.1 | 39.3 ± 1.5 | 0.003 | |
| Body weight, g | 2302 ± 491 | 2611 ± 538 | 0.008 | 0.04 |
| Body length, cm | 44.4 ± 2.3 | 45.8 ± 3.1 | 0.048 | 0.36 |
| Head circumference, cm | 31.5 ± 1.8 | 31.9 ± 1.6 | 0.098 | 0.30 |
| p-Value | ||||
|---|---|---|---|---|
| 24 Months Old | Period-I | Period-II | Unadjusted | Adjusted |
| Numbers | 30 | 32 | ||
| Anthropometry | ||||
| Body weight, kg | 10.8 ± 1.3 | 11.2 ± 1.9 | 0.318 | 0.374 |
| Body length, cm | 84.7 ± 3 | 84.5 ± 4 | 0.688 | 0.923 |
| Head circumference, cm | 46.5 ± 1.5 | 46.1 ± 1.6 | 0.375 | 0.515 |
| BSID-III | ||||
| Cognitive scores | 85.5 ± 11.8 | 88.0 ± 10.6 | 0.394 | 0.150 |
| Language scores | 83.3 ± 12.3 | 87.3 ± 10.5 | 0.178 | 0.048 |
| Motor scores | 81.7 ± 14.1 | 87.5 ± 10.3 | 0.070 | 0.032 |
| Cognitive scores 85, N (%) | 8 | 9 | 1.000 | |
| Language scores 85, N (%) | 14 | 11 | 0.438 | |
| Motor scores 85, N (%) | 11 | 9 | 0.589 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Chen, Y.-J.; Huang, C.-C.; Shieh, C.-C. Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes. Nutrients 2020, 12, 2229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082229
Lin Y-C, Chen Y-J, Huang C-C, Shieh C-C. Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes. Nutrients. 2020; 12(8):2229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082229
Chicago/Turabian StyleLin, Yung-Chieh, Yen-Ju Chen, Chao-Ching Huang, and Chi-Chang Shieh. 2020. "Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes" Nutrients 12, no. 8: 2229. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082229






