Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Sample
2.2. Mice
2.3. Induction of AD and Oral Administration of SHE
2.4. Measurement of Skin Severity Score
2.5. Measurement of Transepidermal Water Loss (TEWL)
2.6. Measurement of Ear Skin Edema
2.7. Histological Observation of Dorsal Back Skin and Ear
2.8. Detection of Cytokine and Chemokine Expression in Dorsal Back Skin and Spleen
2.9. Measurement of Total Serum IgG1 and IgG2a
2.10. Measurement of Cyokines Production in Serum
2.11. Statistics
3. Results
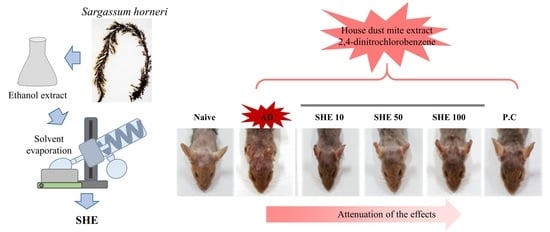
3.1. Oral Administration of SHE Improved the AD Symptoms in HDM/DNCB-Stimulated NC/Nga Mice
3.2. Oral Administration of SHE Decreased the Dryness of the AD Dorsal Skin in the HDM/DNCB-Stimulated NC/Nga Mice
3.3. Oral Administration of SHE Reduced the Ear Edema in HDM/DNCB-Stimulated NC/Nga Mice
3.4. Oral Administration of SHE Decreased the Serum IgG1 and IgG2a levels in HDM/DNCB-Stimulated NC/Nga Mice
3.5. Oral Administration of SHE Inhibited the Hyperkeratosis and Epidermal Hyperplasia with the Reduction of the Infiltrated Mast Cells and Eosinophils in AD Dorsal skin and Ear Lesions of HDM/DNCB-Stimulated NC/Nga Mice
3.6. Oral Administration of SHE Downregulated the mRNA Expression Levels of Cytokines and Chemokines in the AD Dorsal Skin and the Spleen of the HDM/DNCB-Stimulated NC/Nga Mice
3.7. Oral Administration of SHE Suppressed the Production of Cytokines in the HDM/DNCB-Stimulated NC/Nga Mice Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, P.J.; Shahidi, F.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from an edible seaweed sargassum horneri using esr spectrometry. J. Food Lipids 2004, 11, 15–27. [Google Scholar] [CrossRef]
- Kim, D.-S.; Sung, N.-Y.; Park, S.-Y.; Kim, G.; Eom, J.; Yoo, J.-G.; Seo, I.-R.; Han, I.-J.; Cho, Y.-B.; Kim, K.-A. Immunomodulating activity of sargassum horneri extracts in raw264. 7 macrophages. J. Nutr. Health 2018, 51, 507–514. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and isolated 6-hydroxy-4, 4, 7a-trimethyl-5, 6, 7, 7a-tetrahydrobenzofuran-2 (4h)-one (htt); lps-induced inflammation attenuation via suppressing nf-κb, mapk and oxidative stress through nrf2/ho-1 pathways in raw 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kim, S.-Y.; Ye, B.-R.; Kim, J.; Ko, S.-C.; Lee, W.W.; Kim, K.-N.; Choi, I.-W.; Jung, W.-K.; Heo, S.-J. Anti-inflammatory effect of apo-9′-fucoxanthinone via inhibition of mapks and nf-kb signaling pathway in lps-stimulated raw 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Lee, H.G.; Herath, K.H.I.N.M.; Jee, Y.; Jeon, Y.-J. The protective effect of sargassum horneri against particulate matter-induced inflammation in lung tissues of an in vivo mouse asthma model. Food Funct. 2019, 10, 7995–8004. [Google Scholar] [CrossRef]
- Roberfroid, M.B. What is beneficial for health? The concept of functional food. Food Chem. Toxicol. 1999, 37, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-C.; Ahn, G.; Yang, X.; Kim, K.-N.; Kang, S.-M.; Lee, S.-H.; Ko, S.-C.; Ko, J.-Y.; Kim, D.; Kim, Y.-T.; et al. Hepatoprotective effects of dieckol-rich phlorotannins from ecklonia cava, a brown seaweed, against ethanol induced liver damage in balb/c mice. Food Chem. Toxicol. 2012, 50, 1986–1991. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Jeon, Y.-J.; Lee, C.J.; Jin, Y.-H.; Baek, N.-I.; Kim, D.; Kang, S.-M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed ecklonia cava kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type a–benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yao, H.-T.; Chiang, M.-T. Red algae (gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J. Food Drug Anal. 2015, 23, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in nc/nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helkowski, W.M.; Ditunno, J.F., Jr.; Boninger, M. Autonomic dysreflexia: Incidence in persons with neurologically complete and incomplete tetraplegia. J. Spinal Cord Med. 2003, 26, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-J.; Jee, Y.; Kang, S.-H.; Jang, J.-H.; Jang, J.-P.; et al. Anti-allergy effect of mojabanchromanol isolated from sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Mihindukulasooriya, S.P.; Kim, H.J.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Oral administration of polyphenol-rich sargassum horneri suppresses particulate matter exacerbated airway inflammation in murine allergic asthma: Relevance to the tlr mediated nf-κb pathway inhibition. J. Funct. Foods 2020, 71, 103991. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Lee, H.G.; Je, J.-G.; Jee, Y.; Jeon, Y.-J. Sargassum horneri (turner) inhibit urban particulate matter-induced inflammation in mh-s lung macrophages via blocking tlrs mediated nf-κb and mapk activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef]
- Won, T.J.; Kim, B.; Lee, Y.; Bang, J.S.; Oh, E.S.; Yoo, J.-S.; Hyung, K.E.; Yoon, J.; Hwang, S.; Park, E.S.; et al. Therapeutic potential of lactobacillus plantarum cjlp133 for house-dust mite-induced dermatitis in nc/nga mice. Cell. Immunol. 2012, 277, 49–57. [Google Scholar] [CrossRef]
- Jung, M.; Lee, T.H.; Oh, H.J.; Kim, H.; Son, Y.; Lee, E.H.; Kim, J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of nf-κb and stat activation. J. Dermatol. Sci. 2015, 79, 252–261. [Google Scholar] [CrossRef]
- Jung, K.; Tanaka, A.; Fujita, H.; Matsuda, A.; Oida, K.; Karasawa, K.; Okamoto, N.; Ohmori, K.; Jee, Y.; Shin, T.; et al. Peroxisome proliferator-activated receptor γ-mediated suppression of dendritic cell function prevents the onset of atopic dermatitis in nc/tnd mice. J. Allergy Clin. Immunol. 2011, 127, 420–429.e426. [Google Scholar] [CrossRef]
- Ahn, G.; Park, E.-J.; Kim, D.-S.; Jeon, Y.-J.; Shin, T.-K.; Park, J.-W.; Woo, H.-C.; Lee, K.-W.; Jee, Y.-H. Anti-inflammatory effects of enzymatic extract from ecklonia cava on tpa-induced ear skin edema. Food Sci. Biotechnol. 2008, 17, 745–750. [Google Scholar]
- Yang, G.; Lee, H.E.; Shin, S.W.; Um, S.H.; Lee, J.D.; Kim, K.-B.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Efficient transdermal delivery of DNA nanostructures alleviates atopic dermatitis symptoms in nc/nga mice. Adv. Funct. Mater. 2018, 28, 1801918. [Google Scholar] [CrossRef]
- Grewe, M.; Bruijnzeel-Koomen, C.A.; Schopf, E.; Thepen, T.; Langeveld-Wildschut, A.G.; Ruzicka, T.; Krutmann, J. A role for th1 and th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today 1998, 19, 359–361. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.; Mitchell, E.B.; Rowntree, S.; Chapman, M.D.; Wilkins, S.R. The role of dust mite allergens in atopic dermatitis. Clin. Exp. Dermatol. 1983, 8, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Traidl, C.; Jugert, F.; Merk, H.; Krieg, T.; Hunzelmann, N. Inhibition of allergic contact dermatitis to dncb but not to oxazolone in interleukin-4-deficient mice. J. Investig. Dermatol. 1999, 112, 476–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Matsuda, A.; Jung, K.; Jang, H.; Ahn, G.; Ishizaka, S.; Amagai, Y.; Oida, K.; Arkwright, P.D.; Matsuda, H. Ultra-pure soft water ameliorates atopic skin disease by preventing metallic soap deposition in nc/tnd mice and reduces skin dryness in humans. Acta Derm. Venereol. 2015, 95, 787–791. [Google Scholar] [CrossRef]
- Tremblay, L.A.; Gadd, J.B.; Northcott, G.L. Steroid estrogens and estrogenic activity are ubiquitous in dairy farm watersheds regardless of effluent management practices. Agric. Ecosyst. Environ. 2018, 253, 48–54. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Lv, F.; Ge, X.; Li, G. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch. Biochem. Biophys. 2018, 650, 22–29. [Google Scholar] [CrossRef]
- Kalicinska, E.; Wojtas, K.; Majda, J.; Zacharski, M.; Skiba, J.; Sliwowski, J.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Expression of sex steroid receptors and aromatase in adipose tissue in different body regions in men with coronary artery disease with and without ischemic systolic heart failure. Aging Male Off. J. Int. Soc. Study Aging Male 2018, 23, 141–153. [Google Scholar] [CrossRef]
- Falguera, V.; Aliguer, N.; Falguera, M. An integrated approach to current trends in food consumption: Moving toward functional and organic products? Food Control 2012, 26, 274–281. [Google Scholar] [CrossRef]
- Kim, M.J.; Choung, S.-Y. Mixture of polyphenols and anthocyanins from vaccinium uliginosum l. Alleviates dncb-induced atopic dermatitis in nc/nga mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 15. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, J.S.; Yager, J.A.; Eyre, P.; Parker, W.M. Morphometric analyses of the skin of dogs with atopic dermatitis and correlations with cutaneous and plasma histamine and total serum ige. Vet. Pathol. 1990, 27, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (il-33, il-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Choi, M.J.; Bak, D.-H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of egf suppresses immune response and protects skin barrier in dncb-induced atopic dermatitis in nc/nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasakawa, T.; Higashi, Y.; Sakuma, S.; Hirayama, Y.; Sasakawa, Y.; Ohkubo, Y.; Goto, T.; Matsumoto, M.; Matsuda, H. Atopic dermatitis-like skin lesions induced by topical application of mite antigens in nc/nga mice. Int. Arch. Allergy Immunol. 2001, 126, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, S.; Volz, T.; Skabytska, Y.; Köberle, M.; Hein, U.; Chen, K.-M.; Guenova, E.; Wölbing, F.; Röcken, M.; Biedermann, T. Toll-like receptor 2 ligands promote chronic atopic dermatitis through il-4–mediated suppression of il-10. J. Allergy Clin. Immunol. 2014, 134, 92–99.e96. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol. 2014, 31, 84–91. [Google Scholar] [CrossRef]
- Behshad, R.; Cooper, K.D.; Korman, N.J. A retrospective case series review of the peroxisome proliferator-activated receptor ligand rosiglitazone in the treatment of atopic dermatitis. Arch. Dermatol. 2008, 144, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Maynard, C.L.; Weaver, C.T. Diversity in the contribution of interleukin-10 to t-cell-mediated immune regulation. Immunol. Rev. 2008, 226, 219–233. [Google Scholar] [CrossRef] [Green Version]






| Primers | Sequence |
|---|---|
| IL-4 | Forward: ACG GAG ATG GAT GTG CCA AAC |
| Reverse: AGC ACC TTG GAA GCC CTA CAG A | |
| IL-5 | Forward: TCA GCT GTG TCT GGG CCA CT |
| Reverse: TTA TGA GTA GGG ACA GGA AGC CTC A | |
| IL-6 | Forward: CCA CTT CAC AAG TCG GAG GCT TA |
| Reverse: GCA AGT GCA TCA TCG TTG TTC ATA C | |
| IL-10 | Forward: GAC CAG CTG GAC AAC ATA CTG CTA A |
| Reverse: GAT AAG GCT TGG CAA CCC AAG TAA | |
| IL-13 | Forward: CAA TTG CAA TGC CAT CTA CAG GAC |
| Reverse: CGA AAC AGT TGC TTT GTG TAG CTG A | |
| IL-25 | Forward: CTC AAC AGC AGG GCC ACT C |
| Reverse: GTC TGT AGG CTG ACG CAG TGT G | |
| IL-33 | Forward: GAT GAG ATG TCT CGG CTG CTT G |
| Reverse: AGC CGT TAC GGA TAT GGT GGT C | |
| IFN-g | Forward: CGG CAC AGT CAT TGA AAG CCT A |
| reverse: GGC ACC ACT AGT TGG TTG TCT TTG | |
| TARC | Forward: TGA GGT CAC TTC AGA TGC TGC |
| Reverse: ACC AAT CTG ATG GCC TTC TTC | |
| RANTES | Forward: GGA GTA TTT CTA CAC CAG CAG CAA G |
| Reverse: GGC TAG GAC TAG AGC AAG CAA TGA C | |
| Eotaxin | Forward: AAC ATG GCG GGC TCT GCT AC |
| Reverse: CCT GCC TTG GGA CAG ATG CT | |
| STAT3 | Forward: GA AGC CGA CCC AGG TGC |
| Reverse: GT CAC GTC TCT GCA GCT TCT | |
| T-bet | Forward: CG GTA CCA GAG CGG CAA GT |
| Reverse: AG CCC CCT TGT TGT TGG TG | |
| GATA3 | Forward: TC TCA CTC TCG AGG CAG CAT GA |
| Reverse: GGT ACC ATC TCG CCG CCA CAG | |
| GAPDH | Forward: CAT CCG TAA AGA CCT CTA GCC AAC |
| Reverse: ATG GAG CCA CCG ATC CAC A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.J.; Fernando, I.P.S.; Kim, H.-S.; Jeon, Y.-J.; Madusanka, D.M.D.; Dias, M.K.H.M.; Jee, Y.; Ahn, G. Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Nutrients 2020, 12, 2482. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082482
Han EJ, Fernando IPS, Kim H-S, Jeon Y-J, Madusanka DMD, Dias MKHM, Jee Y, Ahn G. Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Nutrients. 2020; 12(8):2482. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082482
Chicago/Turabian StyleHan, Eui Jeong, Ilekuttige Priyan Shanura Fernando, Hyun-Soo Kim, You-Jin Jeon, Dissanayaka Mudiyanselage Dinesh Madusanka, Mawalle Kankanamge Hasitha Madhawa Dias, Youngheun Jee, and Ginnae Ahn. 2020. "Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice" Nutrients 12, no. 8: 2482. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12082482