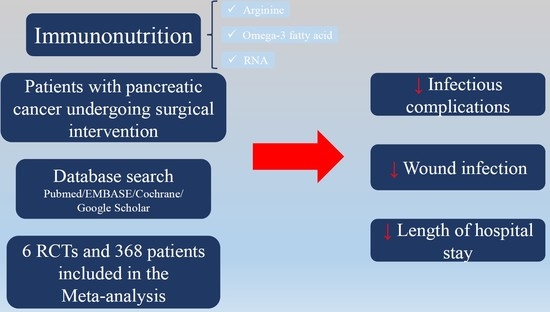
Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.3. Data Items
2.4. Risk-of-Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Risk-of-Bias Assessment
3.4. Effect of Immunonutrition on Postoperative Total Complications
3.5. Effect of Immunonutrition on Postoperative Infectious Complications
3.6. Effect of Immunonutrition on Postoperative Infectious Complications—Wound Infection
3.7. Effect of Immunonutrition on Postoperative Noninfectious Complications
3.8. Effect of Immunonutrition on Postoperative Noninfectious Complications—Delayed Gastric Emptying
3.9. Effect of Immunonutrition on Postoperative Noninfectious Complications—Fistula Development
3.10. Effect of Immunonutrition on Postoperative Mortality
3.11. Effect of Immunonutrition on Length of Hospital Stay
3.12. Effect of Immunonutrition on Postoperative Immunity
4. Discussion
- (1)
- infectious complication, for overall and preoperative group in subgroup analysis
- (2)
- wound infection, for overall analysis
- (3)
- length of hospital stay, for overall and preoperative group in subgroup analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, S.; Van Buren, G., II; Fisher, W.E. Pancreatic cancer: Advances in treatment. World J. Gastroenterol. 2014, 20, 9354–9360. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant Chemotherapy with Gemcitabine vs. Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer A Randomized Controlled Trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef]
- Ueno, H.; Kosuge, T.; Matsuyama, Y.; Yamamoto, J.; Nakao, A.; Egawa, S.; Doi, R.; Monden, M.; Hatori, T.; Tanaka, M.; et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br. J. Cancer 2009, 101, 908–915. [Google Scholar] [CrossRef]
- Mayo, S.C.; Nathan, H.; Cameron, J.L.; Olino, K.; Edil, B.H.; Herman, J.M.; Hirose, K.; Schulick, R.D.; Choti, M.A.; Wolfgang, C.L.; et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012, 118, 2674–2681. [Google Scholar] [CrossRef] [Green Version]
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006, 244, 10–15. [Google Scholar] [CrossRef]
- Leandro-Merhi, V.A.; de Aquino, J.L. Determinants of Malnutrition and Post-operative Complications in Hospitalized Surgical Patients. J. Health Popul. Nutr. 2014, 32, 400–410. [Google Scholar]
- Zhang, Y.; Gu, Y.; Guo, T.; Li, Y.; Cai, H. Perioperative immunonutrition for gastrointestinal cancer: A systematic review of randomized controlled trials. Surg. Oncol. 2012, 21, e87–e95. [Google Scholar] [CrossRef]
- Okamoto, Y.; Okano, K.; Izuishi, K.; Usuki, H.; Wakabayashi, H.; Suzuki, Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J. Surg. 2009, 33, 1815–1821. [Google Scholar] [CrossRef]
- Moskovitz, D.N.; Kim, Y.-I. Does Perioperative Immunonutrition Reduce Postoperative Complications in Patients with Gastrointestinal Cancer Undergoing Operations? Nutr. Rev. 2004, 62, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, F.; Luo, B.; Wu, X. Application of perioperative immunonutrition for gastrointestinal surgery: A meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2007, 16, 253–257. [Google Scholar] [PubMed]
- Cheng, Y.; Zhang, J.; Zhang, L.; Wu, J.; Zhan, Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 11. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [Updated July 2019]. Available online: www.training.cochrane.org/handbook> (accessed on 17 January 2020).
- EndNote X9. Available online: https://endnote.com/ (accessed on 17 January 2020).
- Gade, J.; Levring, T.; Hillingso, J.; Hansen, C.P.; Andersen, J.R. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer—A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef]
- Hamza, N. Perioperative Enteral Immunonutrition Modulates Systemic and Mucosal Immunity and the Inflammatory Response in Patients with Periampullary Cancer Scheduled for Pancreaticoduodenectomy. Pancreas 2015, 44, 41–52. [Google Scholar] [CrossRef]
- Carlo, V.D. Complications of pancreatic surgery and the role of perioperative nutrition. Dig. Surg. 1999, 16, 320–326. [Google Scholar] [CrossRef]
- Gianotti, L.; Braga, M.; Gentilini, O.; Balzano, G.; Zerbi, A.; di Carlo, V. Artificial Nutrition after Pancreaticoduodenectomy. Pancreas 2000, 21, 344–351. [Google Scholar] [CrossRef]
- Słotwiński, R.; Olszewski, W.; Lech, G.; Gulak, G.; Słotwińska, S.M. Immunonutrition after major pancreatic surgery. Cent. Eur. J. Immunol. 2008, 33, 67–73. [Google Scholar]
- Wong, C.S.; Aly, E.H. The effects of enteral immunonutrition in upper gastrointestinal surgery: A systematic review and meta-analysis. Int. J. Surg. 2016, 29, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Akbarshahi, H.; Andersson, B.; Norden, M.; Andersson, R. Perioperative nutrition in elective gastrointestinal surgery—Potential for improvement? Dig. Surg. 2008, 25, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Carrott, P.W.; Patel, J.; Kiraly, L.; Martindale, R.G. Parenteral or Enteral Arginine Supplementation Safety and Efficacy. J. Nutr. 2016, 146, 2594S–2600S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evoy, D.; Lieberman, M.D.; Fahey, T.J., III; Daly, J.M. Immunonutrition: The Role of Arginine. Nutrition 1998, 14, 611–617. [Google Scholar] [CrossRef]
- Drover, J.W.; Dhaliwal, R.; Weitzel, L.; Wischmeyer, P.E.; Ochoa, J.B.; Heyland, D.K. Perioperative use of arginine-supplemented diets: A systematic review of the evidence. J. Am. Coll. Surg. 2011, 212, 385–399. [Google Scholar] [CrossRef]
- Tsekos, E.; Reuter, C.; Stehle, P.; Boeden, G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin. Nutr. 2004, 23, 325–330. [Google Scholar] [CrossRef]
- Wu, G.H.; Zhang, Y.W.; Wu, Z.H. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J. Gastroenterol. 2001, 3, 357–362. [Google Scholar] [CrossRef]
- Alexander, J.W.; Saito, H.I.; Trocki, O.; Ogle, C.K. The Importance of Lipid Type in the Diet after Burn Injury. Ann. Surg. 1986, 204, 1. [Google Scholar] [CrossRef]
- Grant, J.P. On Enteral Nutrition During Multimodality Therapy in Upper Gastrointestinal Cancer Patients. Ann. Surg. 1995, 221, 325–326. [Google Scholar] [CrossRef]
- Manhart, N.; Vierlinger, K.; Akomeah, R.; Bergmeister, H.; Spittler, A.; Roth, E. Influence of enteral diets supplemented with key nutrients on lymphocyte subpopulations in Peyer’s patches of endotoxin-boostered mice. Clin. Nutr. 2000, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, K.A.; Wu, Y.; Fukatsu, K.; Zarzaur, B.L.; Johnson, C.D.; Wang, R.; Hanna, M.K. Glutamine-Enriched Total Parenteral Nutrition Maintains Intestinal Interleukin-4 and Mucosal Immunoglobulin A Levels. J. Parenter. Enteral. Nutr. 2000, 24, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Bao, J.; Yue, T.; Liu, W.; Zhang, Q.; Zhou, L.; Xu, H.J.; Dai, S.M. Secondary failure to treatment with recombinant human IL-1 receptor antagonist in Chinese patients with rheumatoid arthritis. Clin. Rheumatol. 2011, 30, 697–701. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, Y.; Jing, D.; Wu, Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J. Surg. 2006, 30, 1284–1289. [Google Scholar] [CrossRef]
- Zhang, J.; Koh, J.; Lu, J.; Thiel, S.; Leong, B.S.; Sethi, S.; He, C.Y.; Ho, B.; Ding, J.L. Local inflammation induces complement crosstalk which amplifies the antimicrobial response. PLoS Pathog. 2009, 5, e1000282. [Google Scholar] [CrossRef]
- Yao, C.; Sakata, D.; Esaki, Y.; Li, Y.; Matsuoka, T.; Kuroiwa, K.; Sugimoto, Y.; Narumiya, S. Prostaglandin E2–EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat. Med. 2009, 15, 633–640. [Google Scholar] [CrossRef] [Green Version]














| Author | Year | Time of Administration | n | Brand of Immunonutrition | Site of the Tumor | Procedure | Intervention Group n | Control Group n | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Gade [18] | 2016 | preoperative | 35 | IMPACT | Including pancreatic tail | Curative surgery for pancreatic cancer | 19 | 16 | (4)(5) |
| Aida [19] | 2014 | preoperative | 50 | IMPACT | Pancreatic head or peri-ampullary | Pancreaticoduodenectomy | 25 | 25 | (2)(3)(5) |
| Hamza [20] | 2015 | perioperative | 30 | IMPACT | Pancreatic head or peri-ampullary | Pancreaticoduodenectomy | 15 | 15 | (6)(7) |
| Slotwinski [23] | 2008 | postoperative | 41 | Stresson | Pancreatic head | Pancreaticoduodenectomy | 19 | 22 | (6)(7) |
| Gianotti [22] | 2000 | postoperative | 144 | IMPACT | Pancreatic head or peri-ampullary | Pylorus-preserving pancreaticoduodenectomy or Whipple resection | 71 | 73 | (1)(2)(3)(4)(5) |
| Carlo [21] | 1999 | postoperative | 68 | IMPACT | Pancreatic head | Pancreaticoduodenectomy | 33 | 35 | (1)(2)(3)(4)(5) |
| Total | 368 | 182 | 186 |
| Outcome | Pre-Operative | Peri-Operative | Post-Operative | Overall |
|---|---|---|---|---|
| Overall complication | - | - | 0.79 (0.56, 1.12) | 0.79 (0.56, 1.12) |
| Infectious complication | 0.47 (0.23, 0.94) * | - | 0.55 (0.26, 1.18) | 0.50 (0.30, 0.84) * |
| Infectious complication—wound infection | 0.36 (0.16, 0.84) * | - | 0.78 (0.18, 3.42) | 0.44 (0.21, 0.91) * |
| Noninfectious complication | 0.88 (0.58, 1.34) | - | 0.93 (0.59, 1.23) | 0.90 (0.66, 1.23) |
| Noninfectious complication—delayed gastric emptying | 1.67 (0.45, 6.24) | - | 1.03 (0.46, 2.27) | 1.17 (0.59, 2.31) |
| Noninfectious complication—fistula | 1.01 (0.24, 4.20) | - | 1.10 (0.52, 2.32) | 1.00 (0.56, 1.80) |
| Mortality | 0.28 (0.01, 6.51) | - | 2.41 (0.36, 16.11) | 1.35 (0.27, 6.88) |
| Length of hospital stay (MD, 95%CI) | −1.90 (−3.78, −0.02) * | - | −3.04 (−7.68, 1.61) | −2.03 (−3.60, −0.45) * |
| CD4+, POD3 (MD, 95%CI) | - | −3.10 (−11.66, 5.46) | −0.45 (−9.12, 8.22) | −1.79 (−7.88, 4.30) |
| CD4+, POD7 (MD, 95%CI) | - | 3.00 (−6.12, 12.12) | 2.02 (−4.16, 8.20) | 2.33 (−2.79, 7.45) |
| CD8+, POD3 (MD, 95%CI) | - | 1.70 (−8.43, 11.83) | 5.00 (−3.11, 13.11) | 3.71 (−2.62, 10.04) |
| CD8+, POD7 (MD, 95%CI) | - | 2.20 (−8.47, 12.87) | 0.80 (−7.12, 8.72) | 1.30 (−5.06, 7.66) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.-A.; Chen, Y.-C.; Tiong, C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092798
Yang F-A, Chen Y-C, Tiong C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020; 12(9):2798. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092798
Chicago/Turabian StyleYang, Fu-An, Yang-Ching Chen, and Cheng Tiong. 2020. "Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 12, no. 9: 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092798