Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Randomization, Blinding, and Intervention Protocol
2.3. Data Collection and Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Portman, D.J.; Gass, M.L. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Chen, A.; Dagur, G.; Suh, Y.; Smith, N.; Cali, B.; Khan, S.A. Genitourinary syndrome of menopause: An overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. AJOG 2016, 215, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.K.; Kang, S.Y.; Chung, Y.J.; Kim, J.H.; Kim, M.R. The Recent Review of the Genitourinary Syndrome of Menopause. J. Menopausal Med. 2015, 21, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2018, 25, 1321–1330. [Google Scholar] [CrossRef] [Green Version]
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013, 20, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Panay, N. Genitourinary syndrome of the menopause–dawn of a new era? Climacteric 2015, 18, 13–17. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Waetjen, L.E. Genitourinary Changes with Aging. Obstet. Gynecol. Clin. N. Am. 2018, 45, 737–750. [Google Scholar] [CrossRef]
- Nappi, R.E.; Palacios, S.; Bruyniks, N.; Particco, M.; Panay, N. EVES Study investigators. The burden of vulvovaginal atrophy on women’s daily living: Implications on quality of life from a face-to-face real-life survey. Menopause 2019, 26, 485–491. [Google Scholar] [CrossRef]
- Islam, R.M.; Bell, R.J.; Davis, S.R. Prevalence of sexual symptoms in relation to menopause in women in Asia: A systematic review. Menopause 2018, 25, 231–238. [Google Scholar] [CrossRef]
- Moyneur, E.; Dea, K.; Derogatis, L.R.; Vekeman, F.; Dury, A.Y.; Labrie, F. Prevalence of depression and anxiety in women newly diagnosed with vulvovaginal atrophy and dyspareunia. Menopause 2020, 27, 134–142. [Google Scholar] [CrossRef]
- Archer, D.F.; Simon, J.A.; Portman, D.J.; Goldstein, S.R.; Goldstein, I. Ospemifene for the treatment of menopausal vaginal dryness, a symptom of the genitourinary syndrome of menopause. Exp. Rev. Endocrinol. Metab. 2019, 14, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, D.L.; Shuster, L.T.; Dockter, T.; Atherton, P.J.; Thielen, J.; Birrell, S.N.; Sood, R.; Griffin, P.; Terstriep, S.A.; Mattar, B.; et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer 2018, 26, 1335–1343. [Google Scholar] [CrossRef]
- Writing Group for the Women’s Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, C.A.; Glaser, S.L.; Uratsu, C.S.; Selby, J.V.; Kushi, L.H.; Herrinton, L.J. Recent declines in hormone therapy utilization and breast cancer incidence: Clinical and population-based evidence. J. Clin. Oncol. 2006, 24, e49–e50. [Google Scholar] [CrossRef]
- Lev-Sagie, A. Vulvar and Vaginal Atrophy: Physiology, Clinical Presentation, and Treatment Considerations. Clin. Obstet. Gynecol. 2015, 58, 476–491. [Google Scholar] [CrossRef]
- Jaisamrarn, U.; Triratanachat, S.; Chaikittisilpa, S.; Grob, P.; Prasauskas, V.; Taechakraichana, N. Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric 2013, 16, 347–355. [Google Scholar] [CrossRef]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Pitsouni, E.; Grigoriadis, T.; Falagas, M.E.; Salvatore, S.; Athanasiou, S. Laser therapy for the genitourinary syndrome of menopause. A systematic review and meta-analysis. Maturitas 2017, 103, 78–88. [Google Scholar] [CrossRef]
- Lima, S.M.; Yamada, S.S.; Reis, B.F.; Postigo, S.; Galvao da Silva, M.A.; Aoki, T. Effective treatment of vaginal atrophy with isoflavone vaginal gel. Maturitas 2013, 74, 252–258. [Google Scholar] [CrossRef]
- Edwards, D.; Panay, N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition? Climacteric 2016, 19, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Archer, D.F. Dehydroepiandrosterone intra vaginal administration for the management of postmenopausal vulvovaginal atrophy. J. Steroid Biochem. Mol. Biol. 2015, 145, 139–143. [Google Scholar] [CrossRef]
- Al-Saqi, S.H.; Uvnas-Moberg, K.; Jonasson, A.F. Intravaginally applied oxytocin improves post-menopausal vaginal atrophy. Post Reprod. Health 2015, 21, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priemel, M.; von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Staud, R. Vitamin D: More than just affecting calcium and bone. Curr. Rheumatol. Rep. 2005, 7, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Skowrońska, P.; Pastuszek, E.; Kuczyński, W.; Jaszczoł, M.; Kuć, P.; Jakiel, G.; Wocławek-Potocka, I.; Łukaszuk, K. The role of vitamin D in reproductive dysfunction in women—A systematic review. Ann. Agric. Environ. Med. 2016, 23, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive endocrinology of vitamin D. Mol. Cell Endocrinol. 2017, 453, 103–112. [Google Scholar] [CrossRef]
- Rad, P.; Tadayon, M.; Abbaspour, M.; Latifi, S.M.; Rashidi, I.; Delaviz, H. The effect of vitamin D on vaginal atrophy in postmenopausal women. Iran J. Nurs. Midwifery Res. 2015, 20, 211–215. [Google Scholar] [PubMed]
- Lee, A.; Lee, M.R.; Lee, H.H.; Kim, Y.S.; Kim, J.M.; Enkhbold, T.; Kim, T.H. Vitamin D Proliferates Vaginal Epithelium through RhoA Expression in Postmenopausal Atrophic Vagina tissue. Mol. Cells 2017, 40, 677–684. [Google Scholar] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Moan, J.; Dahlback, A.; Porojnicu, A.C. At what time should one go out in the sun? Adv. Exp. Med. Biol. 2008, 624, 86–88. [Google Scholar]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [Green Version]
- Daly, R.M.; Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Sikaris, K.A.; Zimmet, P.Z.; Ebeling, P.R.; Shaw, J.E. Prevalence of vitamin D deficiency and its determinants in Australian adults age 25 years and older: A national, population-based study. Clin. Endocrinol. 2012, 77, 26–35. [Google Scholar] [CrossRef]
- Man, R.E.; Li, L.J.; Cheng, C.Y.; Wong, T.Y.; Lamoureux, E.; Sabanayagam, C. Prevalence and Determinants of Suboptimal Vitamin D Levels in a Multiethnic Asian Population. Nutrients 2017, 9, 313. [Google Scholar] [CrossRef]
- Siwamogsatham, O.; Ongphiphadhanakul, B.; Tangpricha, V. Vitamin D deficiency in Thailand. J. Clin. Transl. Endocrinol. 2014, 2, 48–49. [Google Scholar] [CrossRef] [Green Version]
- Bernard, R. Fundamental of Biostatistics, 5th ed.; Thomson Learning: Duxberry, South Africa, 2000. [Google Scholar]
- Yildirim, B.; Kaleli, B.; Duzcan, E.; Topuz, O. The effects of postmenopausal Vitamin D treatment on vaginal atrophy. Maturitas 2004, 49, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bala, R.; Nagpal, M. Role of Vitamin D in urogenital health of geriatric participants. J. Mid-Life Health 2017, 8, 28–35. [Google Scholar]
- Keshavarzi, Z.; Janghorban, R.; Alipour, S.; Tahmasebi, S.; Jokar, A. The effect of vitamin D and E vaginal suppositories on tamoxifen-induced vaginal atrophy in women with breast cancer. Support Care Cancer 2019, 27, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Riazi, H.; Ghazanfarpour, M.; Taebi, M.; Abdolahian, S. Effect of Vitamin D on the Vaginal Health of Menopausal Women: A Systematic Review. J. Menopausal Med. 2019, 25, 109–116. [Google Scholar] [CrossRef]



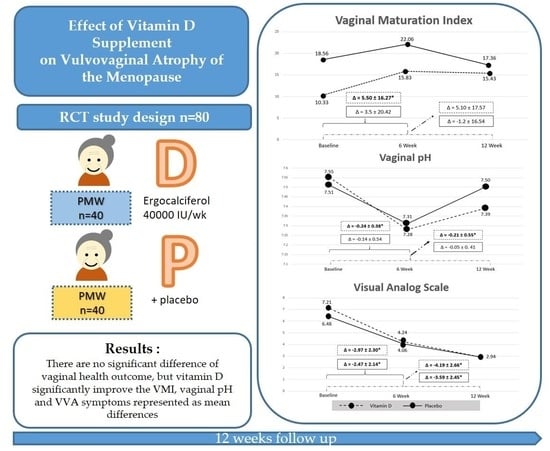
| Characteristics | Vitamin D (N = 40) | Placebo (N = 40) |
|---|---|---|
| Age (years) a | 59.95 ± 5.81 | 58.33 ± 6.25 |
| Age at menopause a | 48.5 ± 5.35 | 49.5 ± 4.24 |
| Body mass index (kg/m2) a | 24.14 ± 3.92 | 24.63 ± 4.24 |
| Active sexual activity b | 19 (51.35%) | 18 (48.65%) |
| Nulliparous b | 7 (17.5%) | 9 (22.5%) |
| Having underlying medical diseases | 20 (50%) | 26 (65%) |
| Smoking history b | 2 (5%) | 0 (0%) |
| Alcohol drinking b | 2 (5%) | 0 (%) |
| Regular exercise b | 22 (44%) | 28 (56%) |
| Vaginal maturation index a | 10.33 ± 19.00 | 18.56 ± 27.99 |
| Vaginal pH a | 7.55 ± 1.02 | 7.51 ± 0.94 |
| VAS of VVA symptoms a | 7.21 ± 2.20 | 6.48 ± 2.28 |
| Serum 25(OH)vitamin D level (ng/mL) a | 24.98 ± 8.25 | 23.28 ± 7.53 |
| Vaginal Health Measurement | Vitamin D | Placebo | p-Value |
|---|---|---|---|
| Vaginal Maturation Index | |||
| Baseline | 10.33 ± 19.00 | 18.56 ± 27.99 | 0.50 |
| Six weeks | 15.83 ± 22.81 | 22.06 ± 28.54 | 0.44 |
| 12 weeks | 15.43 ± 24.87 | 17.36 ± 26.73 | 0.81 |
| Vaginal pH | |||
| Baseline | 7.55 ± 1.02 | 7.51 ± 0.94 | 0.86 |
| Six weeks | 7.28 ± 0.94 | 7.31 ± 0.92 | 0.89 |
| 12 weeks | 7.39 ± 0.96 | 7.50 ± 0.8 | 0.62 |
| VAS of VVA symptoms | |||
| Baseline | 7.21 ± 2.20 | 6.48 ± 2.28 | 0.15 |
| Six weeks | 4.24 ± 2.44 | 4.06 ± 2.47 | 0.76 |
| 12 weeks | 2.94 ± 2.36 | 2.94 ± 2.47 | 0.99 |
| Mean Difference | Vitamin D | p-Value | Placebo | p-Value |
|---|---|---|---|---|
| Vaginal Maturation Index | ||||
| Baseline and at six weeks | 5.5 ± 16.27 * | 0.04 | −3.5 ± 20.42 | 0.29 |
| Baseline and at 12 weeks | 5.1 ± 17.57 | 0.07 | −1.2 ± 16.54 | 0.65 |
| Vaginal pH | ||||
| Baseline and at six weeks | −0.24 ± 0.38 * | <0.05 | −0.14 ± 0.54 | 0.15 |
| Baseline and at 12 weeks | −0.21 ± 0.55 * | 0.03 | −0.05 ± 0.41 | 0.52 |
| VAS of VVA symptoms | ||||
| Baseline and at six weeks | −2.97 ± 2.30 * | <0.01 | −2.47 ± 2.14 * | <0.01 |
| Baseline and at 12 weeks | −4.19 ± 2.66 * | <0.01 | −3.59 ± 2.45 * | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamronrithisorn, T.; Manonai, J.; Vallibhakara, S.A.-O.; Sophonsritsuk, A.; Vallibhakara, O. Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause. Nutrients 2020, 12, 2876. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092876
Kamronrithisorn T, Manonai J, Vallibhakara SA-O, Sophonsritsuk A, Vallibhakara O. Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause. Nutrients. 2020; 12(9):2876. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092876
Chicago/Turabian StyleKamronrithisorn, Thawinee, Jittima Manonai, Sakda Arj-Ong Vallibhakara, Areepan Sophonsritsuk, and Orawin Vallibhakara. 2020. "Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause" Nutrients 12, no. 9: 2876. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12092876