Discovery of Natural Inhibitors of Cholinesterases from Hydrangea: In Vitro and In Silico Approaches
Abstract
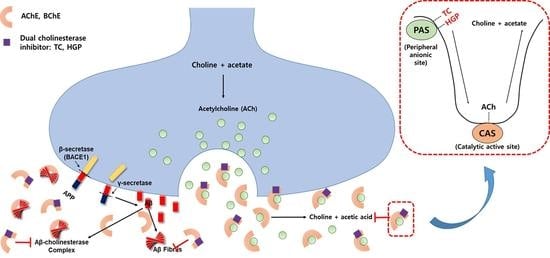
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cholinesterases Inhibition Assay, Enzyme Selectivity and Kinetic Study
2.3. In Silico Docking Analysis
2.4. Statistics
3. Results
3.1. Cholinesterase Inhibitory Activity of Hydrangea-Derived Compounds
3.2. Evaluation of Inhibition Kinetics of Hydrangea-Derived Compounds
3.3. Molecular Interaction Mechanism of Cholinesterases and Hydrangea-Derived Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afshari, A.R.; Sadeghnia, H.R.; Mollazadeh, H. A review on potential mechanisms of Terminalia chebula in Alzheimer’s disease. Adv. Pharmacol. Sci. 2016, 2016, 1–14. [Google Scholar]
- Contestabile, A. The history of the cholinergic hypothesis. Behav. Brain Res. 2011, 221, 334–340. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Li, B.; Stribley, J.A.; Ticu, A.; Xie, W.; Schopfer, L.M.; Hammond, P.; Brimijoin, S.; Hinrichs, S.H.; Lockridge, O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J. Neurochem. 2000, 75, 1320–1331. [Google Scholar] [CrossRef]
- Geula, C.; Darvesh, S. Butyrylcholinesterase, Cholinergic Neurotransmission and the pathology of Alzheimer’s disease. Drugs Today (Barc.) 2004, 40, 711–721. [Google Scholar] [CrossRef]
- Arendt, T.; Brückner, M.K.; Lange, M.; Volker, B. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development-a study of molecular forms. Neurochem. Int. 1992, 21, 381–396. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef]
- Bourne, Y.; Taylor, P.; Radić, Z.; Marchot, P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003, 22, 1–12. [Google Scholar] [CrossRef]
- Galdeano, C.; Viayna, E.; Arroyo, P.; Bidon-Chanal, A.; Blas, J.R.; Muñoz-Torrero, D.; Luque, F.J. Structural determinants of the multifunctional profile of dual binding site acetylcholinesterase inhibitors as anti-Alzheimer agents. Curr. Pharm. Des. 2010, 16, 2818–2836. [Google Scholar] [CrossRef] [PubMed]
- Rees, T.; Hammond, P.I.; Soreq, H.; Younkin, S.; Brimijoin, S. Acetylcholinesterase promotes β-amyloid plaques in cerebral cortex. Neurobiol. Aging 2003, 24, 777–78710. [Google Scholar] [CrossRef]
- Alvarez, A.; Opazo, C.; Alarcon, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-β-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: Therapeutic relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Darvesh, S.; Cash, M.K.; Reid, G.A.; Martin, E.; Mitnitski, A.; Geula, C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Mesulam, M.M.; Geula, C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann. Neurol. 1994, 36, 722. [Google Scholar] [CrossRef]
- Guillozet, A.L.; Smiley, J.F.; Marsh, D.C.; Mesulam, M.M. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Pacheco-Herrero, M.; Thyssen, D.; Murillo-Carretero, M.I.; Berrocoso, E.; Spires-Jones, T.L.; Bacskai, B.J.; Garcia-Alloza, M. Rapid beta-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 2013, 72, 272–285. [Google Scholar] [CrossRef]
- Mori, F.; Lai, C.C.; Fusi, F.; Giacobini, E. Cholinesterase inhibitors increase secretion of APPs in rat brain cortex. Neuroreport 1995, 6, 633–636. [Google Scholar] [CrossRef]
- Cisse, M.; Braun, U.; Leitges, M.; Fisher, A.; Pages, G.; Checler, F.; Vincent, B. ERK1-independent α-secretase cut of β-amyloid precursor protein via M1 muscarinic receptors and PKCα/ε. Mol. Cell. Neurosci. 2011, 47, 223–232. [Google Scholar] [CrossRef]
- Zehnter, R.; Gerlach, H. Enantiodifferentiation in taste perception of the phyllodulcins. Tetrahedron Asymmetry 1995, 6, 2779–2786. [Google Scholar] [CrossRef]
- Zhang, H.; Matsuda, H.; Yamashita, C.; Nakamura, S.; Yoshikawa, M. Hydrangeic acid from the processed leaves of Hydrangea macrophylla var. thunbergii as a new type of anti-diabetic compound. Eur. J. Pharmacol. 2009, 606, 255–261. [Google Scholar] [CrossRef]
- Kurume, A.; Kamata, Y.; Yamashita, M.; Wang, Q.; Matsuda, H.; Yoshikawa, M.; Kawasaki, I.; Ohta, S. Synthesis of 3-substituted isocoumarins and their inhibitory effects on degranulation of RBL-2H3 cells induced by antigen. Chem. Pharm. Bull. 2008, 56, 1264–1269. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Wang, Q.; Matsuhira, K.; Nakamura, S.; Yuan, D.; Yoshikawa, M. Inhibitory effects of thunberginols A and B isolated from Hydrangeae dulcis folium on mRNA expression of cytokines and on activation of activator protein-1 in RBL-2H3 cells. Phytomedicine 2008, 15, 177–184. [Google Scholar] [CrossRef]
- Wang, Q.; Matsuda, H.; Matsuhira, K.; Nakamura, S.; Yuan, D.; Yoshikawa, M. Inhibitory effects of thunberginols A, B, and F on degranulations and releases of TNF-α and IL-4 in RBL-2H3 cells. Biol. Pharm. Bull. 2007, 30, 388–392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Matsuda, H.; Kumahara, A.; Ito, Y.; Nakamura, S.; Yoshikawa, M. New type of anti-diabetic compounds from the processed leaves of Hydrangea macrophylla var. thunbergii (Hydrangeae Dulcis Folium). Bioorg. Med. Chem. Lett. 2007, 17, 4972–4976. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tori, M.; Asakawa, Y. Three dihydroisocoumarin glucosides from Hydrangea macrophylla subsp. serrata. Phytochemistry 1987, 26, 3323–3330. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and cholinesterase inhibitory effects of phlorotannins from Ecklonia cava-An in vitro and in silico study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In silico docking and in vitro spproaches towards BACE1 and cholinesterases inhibitory effect of citrus flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [Green Version]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef] [Green Version]
- Morán, M.A.; Mufson, E.J.; Gomez-Ramos, P. Colocalization of cholinesterases with beta amyloid protein in aged and Alzheimer’s brains. Acta Neuropathol. (Berl.) 1993, 85, 362–369. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [Green Version]
- Welt, T.; Kulic, L.; Hoey, S.E.; McAfoose, J.; Späni, C.; Chadha, A.S.; Fisher, A.; Nitsch, R.M. Acute effects of Muscarinic M1 receptor modulation on AβPP metabolism and Amyloid-β levels in vivo: A Microdialysis study. J. Alzheimers Dis. 2015, 46, 971–982. [Google Scholar] [CrossRef]
- Beaulieu, J.M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J. Psychiatry Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Lauretti, E.; Praticò, D. Caspase-3-dependent cleavage of Akt modulates tau phosphorylation via GSK3β kinase: Implications for Alzheimer’s disease. Mol. Psychiatry 2017, 22, 1002–1008. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally occurring anticholinesterases inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frange, Ž.R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]





| Compounds | AChE | BChE | ||||
|---|---|---|---|---|---|---|
| IC50 1 | Ki Value 2 | Inhibition Type 3 | IC50 1 | Ki Value 2 | Inhibition Type 3 | |
| TC 4 | 41.96 ± 1.06 | 45.6 | Non-competitive | 42.36 ± 3.67 | 49.2 | Non-competitive |
| HGP 5 | 22.66 ± 1.63 | 36.1 | Non-competitive | 41.02 ± 3.03 | 44.9 | Non-competitive |
| Galantamine 6 | 1.72 ± 0.13 | - 7 | Competitive | 12.21 ± 0.55 | - | Competitive |
| Compounds (µM) | BACE1 | Trypsin | Chymotrypsin | Elastase | |
|---|---|---|---|---|---|
| TC | 50 | 25.81 ± 3.11 | −0.27 ± 1.51 | 6.04 ± 2.42 | 1.89 ± 1.09 |
| 100 | 28.68 ± 1.87 | 0.07 ± 2.00 | 8.50 ± 1.40 | 0.42 ± 0.36 | |
| HGP | 50 | 20.02 ± 0.52 | 1.26 ± 3.36 | 7.16 ± 3.38 | 3.77 ± 1.66 |
| 100 | 25.21 ± 1.60 | 3.12 ± 1.69 | 5.15 ± 3.70 | 1.47 ± 1.31 | |
| Enzymes | Ligands | Free Energy (kcal/mol) | No. of H-Bond | Residues | Bond Distance (Å) | van der Waals Residues |
|---|---|---|---|---|---|---|
| AChE | TC | −6.78 | - | TRP286, LEU289, SER293, VAL294, PHE295, PHE297, PHE338, TYR341 | ||
| HGP | −9.77 | 3 | TYR124, TYR337 | 3.16/3.34 3.15 | TYR72, ASP74, THR83, TRP86, GLY121, GLY122, TYR124, SER203, TRP286, PHE295, PHE297, TYR337, TYR341, PHE338, HIS447 | |
| BChE | TC | −7.87 | 1 | GLN67 | 2.99 | ASP70, TRP82, ASN83, PRO84, THR120, GLU197, GLY439, ILE442 |
| HGP | −0.96 | ASN68, LEU274, GLU276, ALA277, PHE278, THR284, VAL280, PRO281, SER287, GLY283, ASN289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Discovery of Natural Inhibitors of Cholinesterases from Hydrangea: In Vitro and In Silico Approaches. Nutrients 2021, 13, 254. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010254
Hwang J, Youn K, Lim G, Lee J, Kim DH, Jun M. Discovery of Natural Inhibitors of Cholinesterases from Hydrangea: In Vitro and In Silico Approaches. Nutrients. 2021; 13(1):254. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010254
Chicago/Turabian StyleHwang, Jayeong, Kumju Youn, Gyutae Lim, Jinhyuk Lee, Dong Hyun Kim, and Mira Jun. 2021. "Discovery of Natural Inhibitors of Cholinesterases from Hydrangea: In Vitro and In Silico Approaches" Nutrients 13, no. 1: 254. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010254