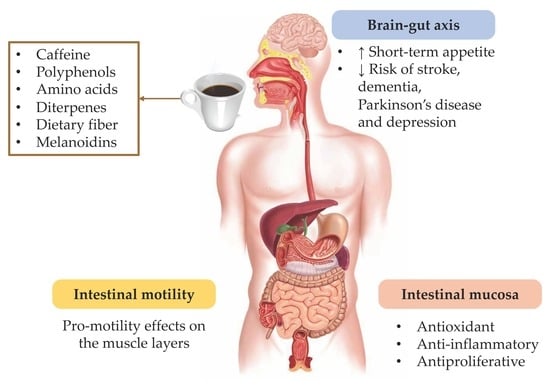
Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis
Abstract
:1. Introduction
2. Coffee and the Gastrointestinal Tract: Focus on the Mucosa
2.1. In Vitro Studies
2.1.1. Coffee
2.1.2. Caffeine
2.1.3. Polyphenols
2.1.4. Diterpenes
2.1.5. Maillard Reaction Products: Melanoidins
2.2. In Vivo Studies
2.2.1. Coffee
2.2.2. Caffeine
2.2.3. Polyphenols
2.2.4. Diterpenes
2.2.5. Maillard Reaction Products: Melanoidins and Acrylamide
3. Coffee and the Gastrointestinal Tract: Focus on Motor Function
3.1. Effects of Coffee Brew on Gastrointestinal Motility
3.2. Caffeine
3.3. Polyphenols
3.4. Dietary Fiber
3.5. Maillard Reaction Products: Melanoidins and Acrylamide
4. Coffee and the Brain–Gut Axis
4.1. Caffeine
4.2. Polyphenols
4.3. Aminoacids and Their Derived Hormones
4.4. Maillard Reaction Products: Melanoidins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [Ca2+]i | intracellular free Ca2+ |
| 5-CQA | 5-O-caffeoylquinic acid |
| ACF | aberrant crypt foci |
| ACE2 | angiotensin-converting enzyme 2 |
| AHP | afterhyperpolarization |
| AKT AP | serine/threonine kinase Akt action potential |
| PKB | protein kinase B |
| ATF-2 | activating transcription factor 2 |
| ATF-3 | activating transcription factor 3 |
| B0AT-1 BBB | sodium-dependent neutral amino acid transporter blood–brain-barrier |
| BE | Barrett’s esophagus |
| CA | caffeic acid |
| Ca2+ cAMP | calcium cyclic adenosine monophosphate |
| CART | cocaine- and amphetamine-regulated transcript |
| CGA | chlorogenic acid |
| CGRP | calcitonin gene-related peptide |
| CICR | calcium-induced calcium release |
| CNS | central nervous system |
| COX-2 | cyclooxygenase-2 |
| CQA | caffeoylquinic acid |
| CRC | colorectal cancer |
| CRP | C-reactive protein |
| DMBA | dimethylbenz(a)anthracene |
| DSS | dextran sodium sulfate |
| EC | enterochromaffin cell |
| EGF ENS | epidermal growth factor enteric nervous system |
| ERK f-EPSP | extracellular signal-regulated kinase fast excitatory postsynaptic potential |
| GABA | γ-aminobutyric acid |
| GERD | gastroesophageal reflux disease |
| GSK3β GST | glycogen synthase kinase 3 beta glutathione S-transferase |
| HE | hematoxylin/eosin |
| HIF-1 | hypoxia-inducible factor-1 |
| HO-1 | heme oxygenase-1 |
| HSP 70 IARC | heat shock protein 70 International Agency for Research on Cancer |
| IBD | inflammatory bowel disease |
| ICC | interstitial cells of Cajal |
| IKK | IkB kinase |
| IL- | interleukin- |
| iNOS | inducible nitric oxide synthase |
| JNK MAPK Mcl-1 MCP-1 | c-Jun N-terminal kinase mitogen-activated protein kinase myeloid cell leukemia 1 methyl-accepting chemotaxis protein-1 |
| SAPK | stress-activated protein kinase |
| MEK MNNG | MAPK/ERK kinase N-methyl-N-nitro-N-nitrosoguanidine |
| MP | myenteric plexus |
| ND | not detected |
| NF-kβ | nuclear factor-kβ |
| nNOS | nitric oxide synthase |
| NO | nitric oxide |
| NR | not reported |
| PAI-1 | plasminogen activator inhibitor-1 |
| PD | Parkinsons’s disease |
| PTEN PG | phosphatase and tensin homolog prostaglandin |
| PhIP | 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine |
| ROS | reactive oxygen species |
| RP | resting potential |
| RyR | ryanodine receptors |
| s-AHP | slow afterhyperpolarization |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SCFA | short chain fatty acid |
| SCG | spent coffee grounds |
| s-EPSP | slow excitatory postsynaptic potential |
| SMP | submucous plexus |
| SP | substance P |
| spp. STAT5 TNF-R | species signal transducer and activator of transcription 5 tumor necrosis factor-receptor |
| TNF | tumor necrosis factor |
| TOPK Trp | lymphokine-activated killer t-cell-originated protein kinase-like protein tryptophan |
| UDP UGT1A | uridine diphosphate UDP glucuronosyltransferases |
| VACHT | vesicular acetylcholine transporter |
| VEGF | vascular endothelial growth factor |
| VIP | vasoactive intestinal peptide |
| WHO | World Health Organization |
| ZO-1 | zonulin-1 |
References
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of Drinking Coffee, Mate, and Very Hot Beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, G.; Stepaniak, U.; Polak, M.; Micek, A.; Topor, R.; Stefler, D.; Szafraniec, K.; Pajak, A. Europe PMC Funders Group Coffee Consumption and Risk of Hypertension in the Polish Arm of the HAPIEE Cohort Study. Eur. J. Clin. Nutr. 2016, 70, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebeskind, D.S.; Sanossian, N.; Fu, K.A.; Wang, H.-J.; Arab, L. The Coffee Paradox in Stroke: Increased Consumption Linked with Fewer Strokes. Nutr. Neurosci. 2016, 19, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee Consumption and Health: Umbrella Review of Meta-Analyses of Multiple Health Outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [Green Version]
- Guallar, E.; Blasco-Colmenares, E.; Arking, D.E.; Zhao, D. Moderate Coffee Intake Can Be Part of a Healthy Diet. Ann. Intern. Med. 2017, 167, 283–284. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Iriondo-Dehond, A.; Iriondo-Dehond, M.; Gonzalez, I.; Medrano, A.; Filip, R.; Uribarri, J. Healthy Eating Recommendations: Good for Reducing Dietary Contribution to the Body’s Advanced Glycation/Lipoxidation End Products Pool? Nutr. Res. Rev. 2020, 1–57. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Martín-Cabrejas, M.A.; Farah, A. Coffee Antioxidants in Chronic Diseases; The Royal Society of Chemistry: London, UK, 2019; ISBN 9781788015028. [Google Scholar]
- Farah, A. Coffee Constituents. Coffee Emerg. Health Eff. Dis. Prev. 2012, 1, 21–58. [Google Scholar]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee Melanoidins: Structures, Mechanisms of Formation and Potential Health Impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Juncker, J. European Commission COMMISSION REGULATION (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, 204, 24–44. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Coffee, tea, cocoa. In Food Chemistry, 4th ed.; Belitz, H.-D., Grosch, W., Schieberle, P., Eds.; Springer: Leipzig, Germany, 2009; pp. 938–951. [Google Scholar]
- Martins, A.C.C.L.; Gloria, M.B.A. Changes on the Levels of Serotonin Precursors—Tryptophan and 5-Hydroxytryptophan—during Roasting of Arabica and Robusta Coffee. Food Chem. 2010, 118, 529–533. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and Serotonin Profiles in Beans of Coffea Species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Fogliano, V.; Pellegrini, N. Coffee, Colon Function and Colorectal Cancer. Food Funct. 2012, 3, 916. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jhon, D.-Y. Changes of the Chlorogenic Acid, Caffeine, Gama-Aminobutyric Acid (GABA) and Antioxdant Activities during Germination of Coffee Bean (Coffea Arabica). Emir. J. Food Agric. 2018, 30, 675. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Gniechwitz, D.; Brueckel, B.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Coffee Dietary Fiber Contents and Structural Characteristics as Influenced by Coffee Type and Technological and Brewing Procedures. J. Agric. Food Chem. 2007, 55, 11027–11034. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and Comparison of Cold Brew and Cold Drip Coffee Extraction Methods. J. Sci. Food Agric. 2019, 99, 391–399. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of Methylxantines, Trigonelline, Nicotinic Acid and Nicotinamide Contents in Brews of Green and Processed Arabica and Robusta Coffee Beans—Influence of Steaming, Decaffeination and Roasting Processes on Coffee Beans. LWT 2020, 125, 109344. [Google Scholar] [CrossRef]
- Rao, N.; Fuller, M.; Grim, M. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Fogliano, V.; Morales, F.J. Estimation of Dietary Intake of Melanoidins from Coffee and Bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Galuch, M.B.; Magon, T.F.S.; Silveira, R.; Nicácio, A.E.; Pizzo, J.S.; Bonafe, E.G.; Maldaner, L.; Santos, O.O.; Visentainer, J.V. Determination of Acrylamide in Brewed Coffee by Dispersive Liquid–Liquid Microextraction (DLLME) and Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS). Food Chem. 2019, 282, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Doello, K.; Ortiz, R.; Alvarez, P.J.; Melguizo, C.; Cabeza, L.; Prados, J. Latest in Vitro and in Vivo Assay, Clinical Trials and Patents in Cancer Treatment Using Curcumin: A Literature Review. Nutr. Cancer 2018, 70, 569–578. [Google Scholar] [CrossRef]
- Mesías, M.; Morales, F.J. Acrylamide in Coffee: Estimation of Exposure from Vending Machines. J. Food Compos. Anal. 2016, 48, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Starbucks Blonde Roast. Available online: https://www.starbucks.com/menu/product/873068625/hot?parent=%2Fdrinks%2Fhot-coffees%2Fbrewed-coffees (accessed on 3 September 2020).
- Starbucks Coffee España Sociedad Limitada. Información Nutricional; Starbucks Coffee España Sociedad Limitada: Madrid, Spain, 2020. [Google Scholar]
- Kang, D.; Lee, H.U.; Davaatseren, M.; Chung, M.S. Comparison of Acrylamide and Furan Concentrations, Antioxidant Activities, and Volatile Profiles in Cold or Hot Brew Coffees. Food Sci. Biotechnol. 2020, 29, 141–148. [Google Scholar] [CrossRef]
- Starbucks Starbucks® Cold Brew Coffee. Available online: https://www.starbucks.com/menu/product/2121255/iced?parent=%2Fdrinks%2Fcold-coffees%2Fcold-brews (accessed on 3 September 2020).
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of Nine Common Coffee Extraction Methods: Instrumental and Sensory Analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What Kind of Coffee Do You Drink? An Investigation on Effects of Eight Different Extraction Methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef]
- Lane, S.; Palmer, J.; Christie, B.; Ehlting, J.; Le, C. Can Cold Brew Coffee Be Convenient? A Pilot Study For Caffeine Content in Cold Brew Coffee Concentrate Using High Performance Liquid Chromatography. Arbutus Rev. 2017, 8, 15–23. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and Antioxidant Activity of Cold Brew Coffee. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, F.M.; Coimbra, M.A. Melanoidins from Coffee Infusions. Fractionation, Chemical Characterization, and Effect of the Degree of Roast. J. Agric. Food Chem. 2007, 55, 3967–3977. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Koloski, N.; Holtmann, G.; Talley, N.J. Is There a Causal Link between Psychological Disorders and Functional Gastrointestinal Disorders? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the Brain-Gut Axis in Functional and Chronic-Inflammatory Gastrointestinal Diseases: A Transdisciplinary Challenge. Psychoneuroendocrinology 2020, 111, 104501. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.; Palsson, O.S.; Pandolfino, J.E. Best Practice Update: Incorporating Psychogastroenterology Into Management of Digestive Disorders. Gastroenterology 2018, 154, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.A.L. Psychogastroenterology: A Cure, Band-Aid, or Prevention? Children 2020, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R. Coffee Consumption vs. Cancer Risk—A Review of Scientific Data. Rocz. Państwowego Zakładu Hig. 2015, 66, 293–298. [Google Scholar]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct. 2014, 5, 695–1717. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Licaj, I.; Lund, E.; Skeie, G.; Weiderpass, E.; Braaten, T. Coffee Consumption and the Risk of Cancer in the Norwegian Women and Cancer (NOWAC) Study. Eur. J. Epidemiol. 2016, 31, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Ma, D.; Zhang, Y.; Zheng, W.; Wang, P. Coffee Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Public Health Nutr. 2013, 16, 346–357. [Google Scholar] [CrossRef]
- Tian, C.; Wang, W.; Hong, Z.; Zhang, X. Coffee Consumption and Risk of Colorectal Cancer: A Dose-Response Analysis of Observational Studies. Cancer Causes Control 2013, 24, 1265–1268. [Google Scholar] [CrossRef]
- Galeone, C.; Turati, F.; La Vecchia, C.; Tavani, A. Coffee Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Case-Control Studies. Cancer Causes Control 2010, 21, 1949–1959. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Yuan, C.; Wu, K.; Smith-Warner, S.A.; Hu, F.B.; Chan, A.T.; Meyerhardt, J.A.; Ogino, S.; Fuchs, C.S.; et al. Association Between Coffee Intake After Diagnosis of Colorectal Cancer and Reduced Mortality. Gastroenterology 2018, 154, 916–926.e9. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tajima, K.; Hirose, K.; Hamajima, N.; Takezaki, T.; Kuroishi, T.; Tominaga, S. Tea and Coffee Consumption and the Risk of Digestive Tract Cancers: Data from a Comparative Case-Referent Study in Japan. Cancer Causes Control 1998, 9, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Meta-Analysis of Coffee Consumption and Risk of Colorectal Cancer. Am. J. Epidemiol. 1998, 147, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee Consumption and the Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Je, Y.; Liu, W.; Giovannucci, E. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Int. J. Cancer 2009, 124, 1662–1668. [Google Scholar] [CrossRef]
- Mackintosh, C.; Yuan, C.; Ou, F.-S.; Zhang, S.; Niedzwiecki, D.; Chang, I.-W.; O’Neil, B.H.; Mullen, B.C.; Lenz, H.-J.; Blanke, C.D.; et al. Association of Coffee Intake with Survival in Patients With Advanced or Metastatic Colorectal Cancer. JAMA Oncol. 2020, 6, 1713. [Google Scholar] [CrossRef]
- Micek, A.; Gniadek, A.; Kawalec, P.; Brzostek, T. Coffee Consumption and Colorectal Cancer Risk: A Dose-Response Meta-Analysis on Prospective Cohort Studies. Int. J. Food Sci. Nutr. 2019, 70, 986–1006. [Google Scholar] [CrossRef]
- Sartini, M.; Bragazzi, N.L.; Spagnolo, A.M.; Schinca, E.; Ottria, G.; Dupont, C.; Cristina, M.L. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 694. [Google Scholar] [CrossRef] [Green Version]
- Dik, V.K.; Bueno-De-Mesquita, H.B.; Van Oijen, M.G.H.; Siersema, P.D.; Uiterwaal, C.S.P.M.; Van Gils, C.H.; Van Duijnhoven, F.J.B.; Cauchi, S.; Yengo, L.; Froguel, P.; et al. Coffee and Tea Consumption, Genotype-Based CYP1A2 and NAT2 Activity and Colorectal Cancer Risk—Results from the EPIC Cohort Study. Int. J. Cancer 2014, 135, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Freedman, N.D.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; Setiawan, V.W. Prospective Study of Coffee Consumption and Cancer Incidence in Non-White Populations. Cancer Epidemiol. Biomark. Prev. 2018, 27, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Terry, P.; Bergkvist, L.; Holmberg, L.; Wolk, A. Coffee Consumption and Risk of Colorectal Cancer in a Population Based Prospective Cohort of Swedish Women. Gut 2001, 49, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Coleman, H.G.; McMenamin, Ú.C.; Cardwell, C.R. Coffee Consumption by Type and Risk of Digestive Cancer: A Large Prospective Cohort Study. Br. J. Cancer 2019, 120, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Huang, S.; He, T.; Su, Y. Coffee Consumption and Risk of Gastric Cancer: An Updated Meta-Analysis. Asia Pac. J. Clin. Nutr. 2016, 25, 578–588. [Google Scholar] [PubMed]
- Bidel, S.; Hu, G.; Jousilahti, P.; Pukkala, E.; Hakulinen, T.; Tuomilehto, J. Coffee Consumption and Risk of Gastric and Pancreatic Cancer—A Prospective Cohort Study. Int. J. Cancer 2013, 132, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Coffee Consumption and Stomach Cancer Risk in a Cohort of Swedish Women. Int. J. Cancer 2006, 119, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.S.; Freedman, N.D.; Kamangar, F.; Dawsey, S.M.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Tea, Coffee, Carbonated Soft Drinks and Upper Gastrointestinal Tract Cancer Risk in a Large United States Prospective Cohort Study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turati, F.; Galeone, C.; La Vecchia, C.; Garavello, W.; Tavani, A. Coffee and Cancers of the Upper Digestive and Respiratory Tracts: Meta-Analyses of Observational Studies. Ann. Oncol. 2011, 22, 536–544. [Google Scholar] [CrossRef]
- Islami, F.; Boffetta, P.; Ren, J.S.; Pedoeim, L.; Khatib, D.; Kamangar, F. High-Temperature Beverages and Foods and Esophageal Cancer Risk—A Systematic Review. Int. J. Cancer 2009, 125, 491–524. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-S.; Yang, J.; Fu, Y.-Q.; Huang, T.; Huang, Y.-J.; Li, D. Effects of Green Tea, Black Tea, and Coffee Consumption on the Risk of Esophageal Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Cancer 2013, 65, 1–16. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.W.; Myung, S.K.; Kwon, H.; Lee, C.; Yun, J.M.; Lee, H.K. Association between Coffee Intake and Gastroesophageal Reflux Disease: A Meta-Analysis. Dis. Esophagus 2014, 27, 311–317. [Google Scholar] [CrossRef]
- Filiberti, R.A.; Fontana, V.; De Ceglie, A.; Blanchi, S.; Grossi, E.; Della Casa, D.; Lacchin, T.; De Matthaeis, M.; Ignomirelli, O.; Cappiello, R.; et al. Association between Coffee or Tea Drinking and Barrett’s Esophagus or Esophagitis: An Italian Study. Eur. J. Clin. Nutr. 2017, 71, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajja, K.C.; El-Serag, H.B.; Thrift, A.P. Coffee or Tea, Hot or Cold, Are Not Associated With Risk of Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2016, 14, 769–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotta, R.B.; Floch, M.H. Diet and Nutrition in Ulcer Disease. Med. Clin. N. Am. 1991, 75, 967–979. [Google Scholar] [CrossRef]
- Cohen, S.; Booth, G.H. Gastric Acid Secretion and Lower-Esophageal-Sphincter Pressure in Response to Coffee and Caffeine. N. Engl. J. Med. 1975, 293, 897–899. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.P.J.; Rezzi, S. Metabolomics View on Gut Microbiome Modulation by Polyphenol-Rich Foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Rubach, M.; Lang, R.; Skupin, C.; Hofmann, T.; Somoza, V. Activity-Guided Fractionation to Characterize a Coffee Beverage That Effectively down-Regulates Mechanisms of Gastric Acid Secretion as Compared to Regular Coffee. J. Agric. Food Chem. 2010, 58, 4153–4161. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [Green Version]
- Dorado, G.; Barbancho, M.; Pueyo, C. Coffee Is Highly Mutagenic in the L-Arabinose Resistance Test InSalmonella Typhimurium. Environ. Mutagen. 1987, 9, 251–260. [Google Scholar] [CrossRef]
- Nagao, M.; Fujita, Y.; Wakabayashi, K. Mutagens in Coffee and Other Beverages. Environ. Health Perspect. 1986, 67, 89–91. [Google Scholar] [CrossRef]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-Inflammatory and Barrier-Stabilising Effects of Myrrh, Coffee Charcoal and Chamomile Flower Extract in a Co-Culture Cell Model of the Intestinal Mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Coffee Induces Expression of Glucuronosyltransferases by the Aryl Hydrocarbon Receptor and Nrf2 in Liver and Stomach. Gastroenterology 2010, 139, 1699–1710.e2. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.A.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an Antioxidant: Inhibition of Lipid Peroxidation Induced by Reactive Oxygen Species. Biochim. Biophys. Actan Biomembr. 1996, 1282, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Antioxidant Ability of Caffeine and Its Metabolites Based on the Study of Oxygen Radical Absorbing Capacity and Inhibition of LDL Peroxidation. Clin. Chim. Acta 2000, 295, 141–154. [Google Scholar] [CrossRef]
- Mitani, T.; Nagano, T.; Harada, K.; Yamashita, Y.; Ashida, H. Caffeine-Stimulated Intestinal Epithelial Cells Suppress Lipid Accumulation in Adipocytes. J. Nutr. Sci. Vitaminol. (Tokyo) 2017, 63, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.K.; Ji, I.M.; Lee, S.R.; Kook, Y.H.; Griffin, R.J.; Lim, B.U.; Kim, J.-S.; Lee, D.S.; Song, C.W.; Park, H.J. Radiosensitization of Tumor Cells by Modulation of ATM Kinase. Int. J. Radiat. Biol. 2006, 82, 277–283. [Google Scholar] [CrossRef]
- Saito, Y.; Gopalan, B.; Mhashilkar, A.M.; Roth, J.A.; Chada, S.; Zumstein, L.; Ramesh, R. Adenovirus-Mediated PTEN Treatment Combined with Caffeine Produces a Synergistic Therapeutic Effect in Colorectal Cancer Cells. Cancer Gene Ther. 2003, 10, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Merighi, S.; Benini, A.; Mirandola, P.; Gessi, S.; Varani, K.; Simioni, C.; Leung, E.; Maclennan, S.; Baraldi, P.G.; Borea, P.A. Caffeine Inhibits Adenosine-Induced Accumulation of Hypoxia-Inducible Factor-1α, Vascular Endothelial Growth Factor, and Interleukin-8 Expression in Hypoxic Human Colon Cancer Cells. Mol. Pharmacol. 2007, 72, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee Phenolic Phytochemicals Suppress Colon Cancer Metastasis by Targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Mhaidat, N.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Alsaad, A.A. Caffeine Inhibits Paclitaxel-Induced Apoptosis in Colorectal Cancer Cells through the Upregulation of Mcl-1 Levels. Mol. Med. Rep. 2014, 9, 243–248. [Google Scholar] [CrossRef]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The Cytotoxicity of Kahweol in HT-29 Human Colorectal Cancer Cells Is Mediated by Apoptosis and Suppression of Heat Shock Protein 70 Expression. Biomol. Ther. (Seoul) 2015, 23, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Huang, W.-Y.; Xiong, X.; Freedman, N.D.; Cross, A.J.; et al. Serum Biomarkers of Habitual Coffee Consumption May Provide Insight into the Mechanism Underlying the Association between Coffee Consumption and Colorectal Cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Chen, Y.; Black, R.M.; Brown, P.H.; Lyle, B.J.; Liu, R.H.; Ou, B. Type 2 Diabetes-Related Bioactivities of Coffee: Assessment of Antioxidant Activity, NF-ΚB Inhibition, and Stimulation of Glucose Uptake. Food Chem. 2011, 124, 914–920. [Google Scholar] [CrossRef]
- Zhao, Z.; Shin, H.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic Acid and Caffeic Acid Down-Regulate the Oxidative Stress- and TNF-α-Induced Secretion of Interleukin-8 from Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, S.K.; Blomhoff, R.; Paur, I. Coffee and Cancer Risk, Epidemiological Evidence, and Molecular Mechanisms. Mol. Nutr. Food Res. 2014, 58, 915–930. [Google Scholar] [CrossRef]
- Murad, L.D.; da Soares, N.C.P.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of Caffeic and 5-Caffeoylquinic Acids on Cell Viability and Cellular Uptake in Human Colon Adenocarcinoma Cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Oleaga, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Coffee Polyphenols Change the Expression of STAT5B and ATF-2 Modifying Cyclin D1 Levels in Cancer Cells. Oxid. Med. Cell. Longev. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA Methylation by Caffeic Acid and Chlorogenic Acid, Two Common Catechol-Containing Coffee Polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, H.; Rogoll, D.; Scheppach, W.; Melcher, R.; Richling, E. The Ussing Type Chamber Model to Study the Intestinal Transport and Modulation of Specific Tight-Junction Genes Using a Colonic Cell Line. Mol. Nutr. Food Res. 2009, 53, 1211–1225. [Google Scholar] [CrossRef]
- Park, G.H.; Song, H.M.; Jeong, J.B. The Coffee Diterpene Kahweol Suppresses the Cell Proliferation by Inducing Cyclin D1 Proteasomal Degradation via ERK1/2, JNK and GKS3β-Dependent Threonine-286 Phosphorylation in Human Colorectal Cancer Cells. Food Chem. Toxicol. 2016, 95, 142–148. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-ΚB: Linking Inflammation and Immunity to Cancer Development and Progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive Effects of the Kahweol and Cafestol on Cyclooxygenase-2 Expression in Macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.J.; Jeong, H.G. Protective Effects of Kahweol and Cafestol against Hydrogen Peroxide-Induced Oxidative Stress and DNA Damage. Toxicol. Lett. 2007, 173, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W.; Schilter, B. Cafestol and Kahweol, Two Coffee Specific Diterpenes with Anticarcinogenic Activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. The Coffee Diterpene Kahweol Induces Heme Oxygenase-1 via the PI3K and P38/Nrf2 Pathway to Protect Human Dopaminergic Neurons from 6-Hydroxydopamine-Derived Oxidative Stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Effect of Dietary Melanoidins on Lipid Peroxidation during Simulated Gastric Digestion: Their Possible Role in the Prevention of Oxidative Damage. J. Agric. Food Chem. 2010, 58, 2513–2519. [Google Scholar] [CrossRef]
- Faist, V.; Erbersdobler, H.F. Metabolic Transit and in Vivo Effects of Melanoidins and Precursor Compounds Deriving from the Maillard Reaction. Ann. Nutr. Metab. 2001, 45, 1–12. [Google Scholar] [CrossRef]
- Würzner, H.-P.; Lindström, E.; Vuataz, L.; Luginbühl, H. A 2-Year Feeding Study of Instant. Coffees in Rats. II. Incidence and Types of Neoplasms. Food Cosmet. Toxicol. 1977, 15, 289–296. [Google Scholar] [CrossRef]
- Stalder, R.; Bexter, A.; Würzner, H.P.; Luginbühl, H. A Carcinogenicity Study of Instant Coffee in Swiss Mice. Food Chem. Toxicol. 1990, 28, 829–837. [Google Scholar] [CrossRef]
- Gershbein, L.L. Action of Dietary Trypsin, Pressed Coffee Oil, Silymarin and Iron Salt on 1,2-Dimethylhydrazine Tumorigenesis by Gavage. Anticancer Res. 1994, 14, 1113–1116. [Google Scholar]
- Chuang, Y.-H.; Quach, A.; Absher, D.; Assimes, T.; Horvath, S.; Ritz, B. Coffee Consumption Is Associated with DNA Methylation Levels of Human Blood. Eur. J. Hum. Genet. 2017, 25, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cibicková, E.; Cibicek, N.; Zd’ánský, P.; Kohout, P. The Impairment of Gastroduodenal Mucosal Barrier by Coffee. Acta Medica Hradec. Kral. 2004, 47, 273–275. [Google Scholar] [CrossRef]
- Cuervo, A.; Hevia, A.; López, P.; Suárez, A.; Diaz, C.; Sánchez, B.; Margolles, A.; González, S. Phenolic Compounds from Red Wine and Coffee Are Associated with Specific Intestinal Microorganisms in Allergic Subjects. Food Funct. 2016, 7, 104–109. [Google Scholar] [CrossRef]
- Nakayama, T.; Oishi, K. Influence of Coffee (Coffea Arabica ) and Galacto-Oligosaccharide Consumption on Intestinal Microbiota and the Host Responses. FEMS Microbiol. Lett. 2013, 343, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of Coffee Consumption on the Gut Microbiota: A Human Volunteer Study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J.; Richoz, J.; Constable, A.; Curtis, K.D.; Dingley, K.H.; Turteltaub, K.W. The Effects of Coffee on Enzymes Involved in Metabolism of the Dietary Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine in Rats. Chem. Biol. Interact. 2003, 145, 251–265. [Google Scholar] [CrossRef]
- Carter, O.; Wang, R.; Dashwood, W.M.; Orner, G.A.; Fischer, K.A.; Löhr, C.V.; Pereira, C.B.; Bailey, G.S.; Williams, D.E.; Dashwood, R.H. Comparison of White Tea, Green Tea, Epigallocatechin-3-Gallate, and Caffeine as Inhibitors of PhIP-Induced Colonic Aberrant Crypts. Nutr. Cancer 2007, 58, 60–65. [Google Scholar] [CrossRef]
- Den Braak, V.; Jong, D.; Rijt, V.; Ruijter, D.; Katan, P. Nagengast The Effect of Unfiltered Coffee on Potential Biomarkers for Colonic Cancer Risk in Healthy Volunteers: A Randomized Trial. Aliment. Pharmacol. Ther. 2000, 14, 1181–1190. [Google Scholar]
- Wang, R.; Dashwood, W.M.; Löhr, C.V.; Fischer, K.A.; Pereira, C.B.; Louderback, M.; Nakagama, H.; Bailey, G.S.; Williams, D.E.; Dashwood, R.H. Protective versus Promotional Effects of White Tea and Caffeine on PhIP-Induced Tumorigenesis and β-Catenin Expression in the Rat. Carcinogenesis 2008, 29, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.V.; Kannen, V.; Junior, A.A.J.; Garcia, S.B. Coffee, but Neither Decaffeinated Coffee nor Caffeine, Elicits Chemoprotection Against a Direct Carcinogen in the Colon of Wistar Rats. Nutr. Cancer 2019, 71, 615–623. [Google Scholar] [CrossRef]
- Nishikawa, A.; Furukawa, F.; Imazawa, T.; Ikezaki, S.; Hasegawa, T.; Takahashi, M. Effects of Caffeine on Glandular Stomach Carcinogenesis Induced in Rats by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Sodium Chloride. Food Chem. Toxicol. 1995, 33, 21–26. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic Acid, Quercetin-3-Rutinoside and Black Tea Phenols Are Extensively Metabolized in Humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.G.; McWhorter, K.; Rivera-Hidalgo, F.; Wright, J.M.; Hirsbrunner, P.; Sunahara, G.I. Kahweol and Cafestol: Inhibitors of Hamster Buccal Pouch Carcinogenesis. Nutr. Cancer 1991, 15, 41–46. [Google Scholar] [CrossRef]
- Huber, W.W.; McDaniel, L.P.; Kaderlik, K.R.; Teitel, C.H.; Lang, N.P.; Kadlubar, F.F. Chemoprotection against the Formation of Colon DNA Adducts from the Food-Borne Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) in the Rat. Mutat. Res. Mol. Mech. Mutagen. 1997, 376, 115–122. [Google Scholar] [CrossRef]
- Lam, L.K.; Sparnins, V.L.; Wattenberg, L.W. Isolation and Identification of Kahweol Palmitate and Cafestol Palmitate as Active Constituents of Green Coffee Beans That Enhance Glutathione S-Transferase Activity in the Mouse. Cancer Res. 1982, 42, 1193–1198. [Google Scholar]
- De Roos, B.; Sawyer, J.K.; Katan, M.B.; Rudel, L.L. Validity of Animal Models for the Cholesterol-Raising Effects of Coffee Diterpenes in Human Subjects. Proc. Nutr. Soc. 1999, 58, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Anton, P.M.; Craus, A.; Niquet-Léridon, C.; Tessier, F.J. Highly Heated Food Rich in Maillard Reaction Products Limit an Experimental Colitis in Mice. Food Funct. 2012, 3, 941. [Google Scholar] [CrossRef]
- Rice, J.M. The Carcinogenicity of Acrylamide. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; La Vecchia, C.; Bosetti, C.; Boyle, P.; Boffetta, P. Exposure to Acrylamide and Human Cancer—A Review and Meta-Analysis of Epidemiologic Studies. Ann. Oncol. 2011, 22, 1487–1499. [Google Scholar] [CrossRef]
- El-Mehi, A.E.; El-Sherif, N.M. Influence of Acrylamide on the Gastric Mucosa of Adult Albino Rats and the Possible Protective Role of Rosemary. Tissue Cell 2015, 47, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The Enteric Nervous System; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schäfer, K. Enteric Glia: S100, GFAP, and Beyond. Anat. Rec. 2019, 302, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M. Gut Pacemaker Cells: The Interstitial Cells of Cajal (ICC). J. Smooth Muscle Res. 2003, 39, 137–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uranga, J.A.; Martínez, V.; Abalo, R. Mast Cell Regulation and Irritable Bowel Syndrome: Effects of Food Components with Potential Nutraceutical Use. Molecules 2020, 25, 4314. [Google Scholar] [CrossRef]
- Thomas, F.B.; Steinbaugh, J.T.; Fromkes, J.J.; Mekhjian, H.S.; Caldwell, J.H. Inhibitory Effect of Coffee on Lower Esophageal Sphincter Pressure. Gastroenterology 1980, 79, 1262–1266. [Google Scholar] [CrossRef]
- Boekema, P.J.; Samsom, M.; van Henegouwen, G.P.B.; Smout, A.J. Coffee and Gastrointestinal Function: Facts and Fiction: A Review. Scand. J. Gastroenterol. 1999, 34, 35–39. [Google Scholar]
- Chang, L.M.; Chen, G.H.; Chang, C.S.; Lien, H.C.; Kao, C.H. Effect of Coffee on Solid-Phase Gastric Emptying in Patients with Non-Ulcer Dyspepsia. Gaoxiong Yi Xue Ke Xue Za Zhi 1995, 11, 425–429. [Google Scholar]
- Lien, H.-C.; Chen, G.-H.; Chang, C.-S.; Kao, C.-H.; Wang, S.-J. The Effect of Coffee on Gastric Emptying. Nucl. Med. Commun. 1995, 16, 923–926. [Google Scholar] [CrossRef]
- Boekema, P.J.; Lo, B.; Samsom, M.; Akkermans, L.M.A.; Smout, A.J.P.M.; Lo, B.; Samsom, M.; Akkermans, L.; Smout, A. The Effect of Coffee on Gastric Emptying and Oro-Caecal Transit Time. Eur. J. Clin. Investig. 2000, 30, 129–134. [Google Scholar] [CrossRef]
- Elta, G.H.; Behler, E.M.; Colturi, T.J. Comparison of Coffee Intake and Coffee-Induced Symptoms in Patients with Duodenal Ulcer, Nonulcer Dyspepsia, and Normal Controls. Am. J. Gastroenterol. 1990, 85, 1339–1342. [Google Scholar]
- Shirlow, M.J.; Mathers, C.D. A Study of Caffeine Consumption and Symptoms: Indigestion, Palpitations, Tremor, Headache and Insomnia. Int. J. Epidemiol. 1985, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Coffee and Nonulcer Dyspepsia. Am. J. Gastroenterol. 1993, 88, 966. [Google Scholar] [PubMed]
- Boekema, P.J.; Samsom, M.; Roelofs, J.M.; Smout, A.J. Effect of Coffee on Motor and Sensory Function of Proximal Stomach. Dig. Dis. Sci. 2001, 46, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Back, C.; Bayless, T.M. Effect of Caffeine on the Human Small Intestine. Gastroenterology 1976, 71, 738–742. [Google Scholar] [CrossRef]
- Wagner, S.M.; Mekhjian, H.S.; Caldwell, J.H.; Thomas, F.B. Effects of Caffeine and Coffee on Fluid Transport in the Small Intestine. Gastroenterology 1978, 75, 379–381. [Google Scholar] [CrossRef]
- Brettholz, E.; Meshkinpour, H. The Effect of Coffee on Mouth To-Cecum Transit Time. Gastroenterology 1985, 88, 1335. [Google Scholar]
- Glatzel, H.; Hackenberg, K. Wirkungen von Koffeinhaltigem Und Koffeinfreiem Kaffee Auf Die Verdauungsfunktion. Med. Klin. 1967, 62, 625–628. [Google Scholar]
- Matzkies, F.; Perisoara, A. Ultrasound Study of the Effects of Coffee on the Motoric Function of the Gallbladder. Fortschr. Med. 1985, 103, 713–714. [Google Scholar]
- Douglas, B.R.; Jansen, J.B.; Tham, R.T.; Lamers, C.B. Coffee Stimulation of Cholecystokinin Release and Gallbladder Contraction in Humans. Am. J. Clin. Nutr. 1990, 52, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.R.; Cann, P.A.; Read, N.W. Effect of Coffee on Distal Colon Function. Gut 1990, 31, 450–453. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.C.; Welcher, K.; Zimmerman, B.; Stumbo, P. Is Coffee a Colonie Stimulant? Eur. J. Gastroenterol. Hepatol. 1998, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Chvasta, T.E.; Cooke, A.R. Emptying and Absorption of Caffeine from the Human Stomach. Gastroenterology 1971, 61, 838–843. [Google Scholar] [CrossRef]
- Gkegkes, I.D.; Minis, E.E.; Iavazzo, C. Effect of Caffeine Intake on Postoperative Ileus: A Systematic Review and Meta-Analysis. Dig. Surg. 2020, 37, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Güngördük, K.; Özdemir, İ.A.; Güngördük, Ö.; Gülseren, V.; Gokçü, M.; Sancı, M. Effects of Coffee Consumption on Gut Recovery after Surgery of Gynecological Cancer Patients: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2017, 216, e1–e145. [Google Scholar] [CrossRef] [Green Version]
- Vather, R.; O’Grady, G.; Bissett, I.P.; Dinning, P.G. Postoperative Ileus: Mechanisms and Future Directions for Research. Clin. Exp. Pharmacol. Physiol. 2014, 41, 358–370. [Google Scholar] [CrossRef]
- Sarawate, C.A.; Lin, S.-J.; Walton, S.M.; Crawford, S.Y.; Goldstein, J.L. Economic Burden of Postoperative Ileus (POI) in Abdominal Surgical Procedures. Gastroenterology 2003, 124, A828. [Google Scholar] [CrossRef]
- Müller, S.A.; Rahbari, N.N.; Schneider, F.; Warschkow, R.; Simon, T.; von Frankenberg, M.; Bork, U.; Weitz, J.; Schmied, B.M.; Büchler, M.W. Randomized Clinical Trial on the Effect of Coffee on Postoperative Ileus Following Elective Colectomy. Br. J. Surg. 2012, 99, 1530–1538. [Google Scholar] [CrossRef]
- Dulskas, A.; Klimovskij, M.; Vitkauskiene, M.; Samalavicius, N.E. Effect of Coffee on the Length of Postoperative Ileus After Elective Laparoscopic Left-Sided Colectomy. Dis. Colon Rectum 2015, 58, 1064–1069. [Google Scholar] [CrossRef]
- Piric, M.; Pasic, F.; Rifatbegovic, Z.; Konjic, F. The Effects of Drinking Coffee While Recovering from Colon and Rectal Resection Surgery. Med. Arch. 2015, 69, 357. [Google Scholar] [CrossRef] [Green Version]
- Büchler, M.W.; Seiler, C.M.; Monson, J.R.T.; Flamant, Y.; Thompson-Fawcett, M.W.; Byrne, M.M.; Mortensen, E.R.; Altman, J.F.B.; Williamson, R. Clinical Trial: Alvimopan for the Management of Post-Operative Ileus after Abdominal Surgery: Results of an International Randomized, Double-Blind, Multicentre, Placebo-Controlled Clinical Study. Aliment. Pharmacol. Ther. 2008, 28, 312–325. [Google Scholar] [CrossRef]
- Bell, T.J.; Poston, S.A.; Kraft, M.D.; Senagore, A.J.; Delaney, C.P.; Techner, L. Economic Analysis of Alvimopan in North American Phase III Efficacy Trials. Am. J. Health Pharm. 2009, 66, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Committee on Military Nutrition Research Pharmacology of Caffeine. In Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Ito, Y.; Osa, T.; Kuriyama, H. Topical Differences of Caffeine Action on the Smooth Muscle Cells of the Guinea Pig Alimentary Canal. Jpn. J. Physiol. 1974, 24, 217–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokutomi, Y.; Tokutomi, N.; Nishi, K. The Properties of Ryanodine-Sensitive Ca 2+ Release in Mouse Gastric Smooth Muscle Cells. Br. J. Pharmacol. 2001, 133, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Domae, K.; Hashitani, H.; Suzuki, H. Regional Differences in the Frequency of Slow Waves in Smooth Muscle of the Guinea-Pig Stomach. J. Smooth Muscle Res. 2008, 44, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hennig, G.W.; Fleming, N.W.; Keef, K.D.; Spencer, N.J.; Ward, S.M.; Sanders, K.M.; Smith, T.K. The Mechanism and Spread of Pacemaker Activity Through Myenteric Interstitial Cells of Cajal in Human Small Intestine. Gastroenterology 2007, 132, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.C.; Yule, D.I.; Mulholland, M.W. Caffeine- and Ryanodine-Sensitive Ca2+ Stores in Cultured Guinea Pig Myenteric Neurons. Am. J. Physiol. Liver Physiol. 1996, 270, G594–G603. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Bayguinov, P.O.; Okamoto, T.; Heredia, D.J.; Smith, T.K. Ca2+ Transients in Myenteric Glial Cells during the Colonic Migrating Motor Complex in the Isolated Murine Large Intestine. J. Physiol. 2012, 590, 335–350. [Google Scholar] [CrossRef]
- Brookes, S.J.H. Classes of Enteric Nerve Cells in the Guinea-Pig Small Intestine. Anat. Rec. 2001, 262, 58–70. [Google Scholar] [CrossRef]
- Furness, J. Intrinsic Primary Afferent Neuronsof the Intestine. Prog. Neurobiol. 1998, 54, 1–18. [Google Scholar] [CrossRef]
- Hillsley, K.; Kenyon, J.L.; Smith, T.K. Ryanodine-Sensitive Stores Regulate the Excitability of AH Neurons in the Myenteric Plexus of Guinea-Pig Ileum. J. Neurophysiol. 2000, 84, 2777–2785. [Google Scholar] [CrossRef]
- Rugiero, F.; Gola, M.; Kunze, W.A.A.; Reynaud, J.; Furness, J.B.; Clerc, N. Analysis of Whole-cell Currents by Patch Clamp of Guinea-pig Myenteric Neurones in Intact Ganglia. J. Physiol. 2002, 538, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, H.-Z.; Gao, N.; Gao, C.; Wang, G.; Wang, X.; Peck, O.C.; Kim, G.; Gao, X.; Xia, Y.; et al. Neuroimmune Interactions in Guinea Pig Stomach and Small Intestine. Am. J. Physiol. Liver Physiol. 2003, 284, G154–G164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, A.W.; Cuthbert, A.W. Neuronal Involvement in Type 1 Hypersensitivity Reactions in Gut Epithelia. Br. J. Pharmacol. 1987, 92, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnette, M.S.; Grous, M. Characterization of the Antigen-Induced Contraction of Colonic Smooth Muscle from Sensitized Guinea Pigs. Am. J. Physiol. Liver Physiol. 1992, 262, G144–G149. [Google Scholar] [CrossRef]
- Wood, J. Allergies and the Brain-in-the-Gut. Clin. Perspect Gastroenterol. 2000, 4, 343–348. [Google Scholar]
- Koshihara, Y.; Neichi, T.; Murota, S.; Lao, A.; Fujimoto, Y.; Tatsuno, T. Caffeic Acid Is a Selective Inhibitor for Leukotriene Biosynthesis. Biochim. Biophys. Acta 1984, 792, 92–97. [Google Scholar]
- Iriondo-DeHond, A.; Cornejo, F.S.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Alonso, S.G.; Andres, M.I.S.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients 2019, 11, 1411. [Google Scholar] [CrossRef] [Green Version]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent Coffee Grounds, an Innovative Source of Colonic Fermentable Compounds, Inhibit Inflammatory Mediators in Vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-Chain Fatty Acids Stimulate Colonic Transit via Intraluminal 5-HT Release in Rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [Green Version]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef] [Green Version]
- De la Cruz, S.T.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Castillo, M.D. del An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriondo-DeHond, A.; Aparicio García, N.; Velazquez Escobar, F.; San Andres, M.I.; Sanchez-Fortun, S.; Blanch, G.P.; Fernandez-Gomez, B.; Guisantes Batan, E.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, M.D.; Martinez-Saez, N.; Amigo-Benavent, M.; Silvan, J.M. Phytochemomics and Other Omics for Permitting Health Claims Made on Foods. Food Res. Int. 2013, 54, 1237–1249. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M.; Srinivas, P. Production of α-Amylase under Solid-State Fermentation Utilizing Coffee Waste. J. Chem. Technol. Biotechnol. 2009, 84, 1246–1249. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional Properties of Coffee and Coffee By-Products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a Potential Functional Food Ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Silván, J.M.; Morales, F.J.; Saura-Calixto, F. Conceptual Study on Maillardized Dietary Fiber in Coffee. J. Agric. Food Chem. 2010, 58, 12244–12249. [Google Scholar] [CrossRef]
- Argirova, M.D.; Stefanova, I.D.; Krustev, A.D.; Turiiski, V.I. Testing Biological Activity of Model Maillard Reaction Products: Studies on Gastric Smooth Muscle Tissues. Amino Acids 2010, 38, 797–803. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard Reaction Products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Health Implications of Acrylamide in Food. Available online: http://apps.who.int/iris/handle/10665/4256 (accessed on 10 November 2020).
- Lourenssen, S.; Miller, K.G.; Blennerhassett, M.G. Discrete Responses of Myenteric Neurons to Structural and Functional Damage by Neurotoxins in Vitro. Am. J. Physiol. Liver Physiol. 2009, 297, G228–G239. [Google Scholar] [CrossRef] [Green Version]
- Belai, A.; Burnstock, G. Acrylamide-Induced Neuropathic Changes in Rat Enteric Nerves: Similarities with Effects of Streptozotocin-Diabetes. J. Auton. Nerv. Syst. 1996, 58, 56–62. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Acrylamide-Induced Alterations in the Cocaine- and Amphetamine-Regulated Peptide Transcript (CART)-like Immunoreactivity within the Enteric Nervous System of the Porcine Small Intestines. Ann. Anat. Anat. Anzeiger 2018, 219, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-X.; Hökfelt, T. The Participation of Galanin in Pain Processing at the Spinal Level. Trends Pharmacol. Sci. 2002, 23, 468–474. [Google Scholar] [CrossRef]
- Locker, F.; Lang, A.A.; Koller, A.; Lang, R.; Bianchini, R.; Kofler, B. Galanin Modulates Human and Murine Neutrophil Activation in Vitro. Acta Physiol. 2015, 213, 595–602. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. Int. J. Mol. Sci. 2019, 20, 3345. [Google Scholar] [CrossRef]
- Palus, K.; Całka, J. Influence of Acrylamide Administration on the Neurochemical Characteristics of Enteric Nervous System (ENS) Neurons in the Porcine Duodenum. Int. J. Mol. Sci. 2019, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Zong, C.; Hasegawa, R.; Urushitani, M.; Zhang, L.; Nagashima, D.; Sakurai, T.; Ichihara, S.; Ohsako, S.; Ichihara, G. Role of Microglial Activation and Neuroinflammation in Neurotoxicity of Acrylamide in Vivo and in Vitro. Arch. Toxicol. 2019, 93, 2007–2019. [Google Scholar] [CrossRef]
- Lyte, J.M. Eating for 3.8 × 1013: Examining the Impact of Diet and Nutrition on the Microbiota-Gut-Brain Axis through the Lens of Microbial Endocrinology. Front. Endocrinol. (Lausanne) 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mulak, A. Brain-Gut-Microbiota Axis in Parkinson’s Disease. World J. Gastroenterol. 2015, 21, 10609. [Google Scholar] [CrossRef]
- Scheperjans, F.; Pekkonen, E.; Kaakkola, S.; Auvinen, P. Linking Smoking, Coffee, Urate, and Parkinson’s Disease—A Role for Gut Microbiota? J. Parkinsons. Dis. 2015, 5, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Kechribari, I.; Sotirakoglou, Κ.; Tarantilis, P.; Gourdomichali, T.; Michas, G.; Kravvariti, V.; Voumvourakis, K.; Zampelas, A. Acute Effects of Coffee Consumption on Self-Reported Gastrointestinal Symptoms, Blood Pressure and Stress Indices in Healthy Individuals. Nutr. J. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 1–21. [Google Scholar]
- Alasmari, F. Caffeine Induces Neurobehavioral Effects through Modulating Neurotransmitters. Saudi Pharm. J. 2020, 28, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Solinas, M.; Ferré, S.; You, Z.-B.; Karcz-Kubicha, M.; Popoli, P.; Goldberg, S.R. Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens. J. Neurosci. 2002, 22, 6321–6324. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Logan, J.; Alexoff, D.; Fowler, J.S.; Thanos, P.K.; Wong, C.; Casado, V.; Ferre, S.; Tomasi, D. Caffeine Increases Striatal Dopamine D2/D3 Receptor Availability in the Human Brain. Transl. Psychiatry 2015, 5, e549. [Google Scholar] [CrossRef] [Green Version]
- Pandolfo, P.; Machado, N.J.; Köfalvi, A.; Takahashi, R.N.; Cunha, R.A. Caffeine Regulates Frontocorticostriatal Dopamine Transporter Density and Improves Attention and Cognitive Deficits in an Animal Model of Attention Deficit Hyperactivity Disorder. Eur. Neuropsychopharmacol. 2013, 23, 317–328. [Google Scholar] [CrossRef]
- Manalo, R.V.M.; Medina, P.M.B. Caffeine Protects Dopaminergic Neurons From Dopamine-Induced Neurodegeneration via Synergistic Adenosine-Dopamine D2-Like Receptor Interactions in Transgenic Caenorhabditis Elegans. Front. Neurosci. 2018, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.R.; McHugh, P.; Holtzman, S. Alcohol & Drug Abuse: Caffeine and Schizophrenia. Psychiatr. Serv. 1998, 49, 1415–1417. [Google Scholar]
- Winston, A.P.; Hardwick, E.; Jaberi, N. Neuropsychiatric Effects of Caffeine. Adv. Psychiatr. Treat. 2005, 11, 432–439. [Google Scholar] [CrossRef]
- Ning, Y.-L.; Yang, N.; Chen, X.; Zhao, Z.-A.; Zhang, X.-Z.; Chen, X.-Y.; Li, P.; Zhao, Y.; Zhou, Y.-G. Chronic Caffeine Exposure Attenuates Blast-Induced Memory Deficit in Mice. Chin. J. Traumatol. 2015, 18, 204–211. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Agostinho, P.M.; Carvalho, R.A.; Cunha, R.A. Caffeine Consumption Prevents Diabetes-Induced Memory Impairment and Synaptotoxicity in the Hippocampus of NONcZNO10/LTJ Mice. PLoS ONE 2012, 7, e21899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, S.; Kim, Y.H.; Seo, H.S. Immediate Decrease in γ -AminoButyric Acid after Caffeine Intake in Adolescents: A Preliminary MRS Study. Investig. Magn. Reson. Imaging 2017, 21, 102–105. [Google Scholar] [CrossRef]
- Jee, H.J.J.; Lee, S.G.G.; Bormate, K.J.J.; Jung, Y.-S.S. Effect of Caffeine Consumption on the Risk for Neurological and Psychiatric Disorders: Sex Differences in Human. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract Are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the Health Benefits of the Bioactive Compounds of Coffee Silverskin Extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Lardeau, A.; Poquet, L. Phenolic Acid Metabolites Derived from Coffee Consumption Are Unlikely to Cross the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2013, 76, 134–138. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2016, 15, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Barros Silva, R.; Santos, N.A.G.; Martins, N.M.; Ferreira, D.A.S.; Barbosa, F.; Oliveira Souza, V.C.; Kinoshita, Â.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic Acid Phenethyl Ester Protects against the Dopaminergic Neuronal Loss Induced by 6-Hydroxydopamine in Rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of Chlorogenic Acid Supplementation in MPTP-Intoxicated Mouse. Front. Pharmacol. 2018, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Ascherio, A.; Zhang, S.M.; Hernán, M.A.; Kawachi, I.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective Study of Caffeine Consumption and Risk of Parkinson’s Disease in Men and Women. Ann. Neurol. 2001, 50, 56–63. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Bai, C.-H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Abbott, R.D.; Petrovitch, H.; White, L.R.; Masaki, K.H.; Tanner, C.M.; Curb, J.D.; Grandinetti, A.; Blanchette, P.L.; Popper, J.S.; Ross, G.W. Frequency of Bowel Movements and the Future Risk of Parkinson’s Disease. Neurology 2001, 57, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.; Del Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The Gut-Brain Axis in Parkinson’s Disease: Possibilities for Food-Based Therapies. Eur. J. Pharmacol. 2017, 817, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Singer, D.; Camargo, S.M.R. Collectrin and ACE2 in Renal and Intestinal Amino Acid Transport. Channels 2011, 5, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Bevins, C.L. Events at the Host-Microbial Interface of the Gastrointestinal Tract. V. Paneth Cell α-Defensins in Intestinal Host Defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 173–176. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 Links Amino Acid Malnutrition to Microbial Ecology and Intestinal Inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Yisireyili, M.; Uchida, Y.; Yamamoto, K.; Nakayama, T.; Cheng, X.W.; Matsushita, T.; Nakamura, S.; Murohara, T.; Takeshita, K. Angiotensin Receptor Blocker Irbesartan Reduces Stress-Induced Intestinal Inflammation via AT1a Signaling and ACE2-Dependent Mechanism in Mice. Brain Behav. Immun. 2018, 69, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Christensen, B.; Lubel, J.S. Letter: Gastrointestinal ACE2, COVID-19 and IBD—Opportunity in the Face of Tragedy? Gastroenterology 2020. [Google Scholar] [CrossRef]
- Camargo, S.M.R.; Vuille-dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and Gut Amino Acid Transport. Clin. Sci. 2020, 134, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Mousa, T.Y.; Mousa, O.Y. Nicotinic Acid Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the Potential Role of Tryptophan as the Precursor of Serotonin and Melatonin for the Aged Sleep-Wake Cycle and Immune Function: Streptopelia Risoria as a Model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The Roles of Peripheral Serotonin in Metabolic Homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Voigt, J.P.; Fink, H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Stasi, C.; Sadalla, S.; Milani, S. The Relationship Between the Serotonin Metabolism, Gut-Microbiota and the Gut-Brain Axis. Curr. Drug Metab. 2019, 20, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary Factors and Fluctuating Levels of Melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef]
- Johns, J. Estimation of Melatonin Blood Brain Barrier Permeability. J. Bioanal. Biomed. 2011, 3, 64–69. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016, 17, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front. Psychol. 2015, 6, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Dina, C.Z.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J.Á. Bioactivity of Food Melanoidins Is Mediated by Gut Microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef]
- Barroso, J.M. Commission Regulation (EU) No 432/2012. Off. J. Eur. Union 2012, 13, 9. [Google Scholar]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of Coffee or Coffee Components on Gut Microbiome and Short-Chain Fatty Acids in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Oseguera-Castro, K.Y.; Madrid, J.A.; Martínez Madrid, M.J.; García, O.P.; Del Castillo, M.D.; Campos-Vega, R. Antioxidant Dietary Fiber Isolated from Spent Coffee (Coffea Arabica L.) Grounds Improves Chronotype and Circadian Locomotor Activity in Young Adults. Food Funct. 2019, 10, 4546–4556. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Arreguín-Campos, A.; Cruz-Medrano, M.A.; del Castillo, B.M.D. Spent Coffee (Coffea Arabica L.) Grounds Promote Satiety and Attenuate Energy Intake: A Pilot Study. J. Food Biochem. 2020, e13204. [Google Scholar] [CrossRef]
- Walker, J.M.; Mennella, I.; Ferracane, R.; Tagliamonte, S.; Holik, A.K.; Hölz, K.; Somoza, M.M.; Somoza, V.; Fogliano, V.; Vitaglione, P. Melanoidins from Coffee and Bread Differently Influence Energy Intake: A Randomized Controlled Trial of Food Intake and Gut-Brain Axis Response. J. Funct. Foods 2020, 72, 104063. [Google Scholar] [CrossRef]
- International Coffee Organization. Crop Year Production by Country; International Coffee Organization: London, UK, 2020. [Google Scholar]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]




| Constituent | Green Coffee Beans (100 g) | Roasted Coffee Beans (100 g) | Filtered Coffee Brew (330 mL) | Cold Brew Coffee (330 mL) |
|---|---|---|---|---|
| Carbohydrates | 9–12.5 g | 38 g | 0 g | 0.1 g |
| Fiber | 46–53 g | 31–38 g | 1.2 g | 0 g |
| Lipids | 15–18 g | 17 g | 0.1 g | 0 g |
| Proteins | 8.5–12 g | 7.5–10 g | 0.1 g | 0.1 g |
| Free amino acids | 0.2–0.8 g | ND | NR | NR |
| Tryptophan | 0.14 g | NR | 0.028 g | NR |
| GABA | 0.11 g | NR | NR | NR |
| Caffeine | 0.8–1.4 g | 1.3 g | 0.244 g | 0.412 g |
| Melatonin | 0.7 mg | 0.9 mg | 0.026 mg | NR |
| Serotonin | 1.3 mg | 0.9 mg | 0.048 mg | NR |
| Trigonelline | 0.6–2.0 g | 1 g | 0.026 g | NR |
| Chlorogenic acids | 4.1–9.2 g | 1.9–2.7 g | 0.009 g | 13.2 g |
| Melanoidins | 0 g | 23 g | 0.6 g | NR |
| Acrylamide | 0 μg | 24.4 μg | 0.6–8.5 μg | 1.4–1.8 μg |
| Ash | 3–5.4 g | 4.5 g | 0.1 g | 0 g |
| References | [9,12,13,14,15,16] | [9,12,14,17] | [12,13,17,18,19,20,21,22,23,24,25,26] | [19,21,27,28,29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo-DeHond, A.; Uranga, J.A.; del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010088
Iriondo-DeHond A, Uranga JA, del Castillo MD, Abalo R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients. 2021; 13(1):88. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010088
Chicago/Turabian StyleIriondo-DeHond, Amaia, José Antonio Uranga, Maria Dolores del Castillo, and Raquel Abalo. 2021. "Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis" Nutrients 13, no. 1: 88. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13010088