The Effects of a Partially Hydrolyzed Formula with Low Lactose and Probiotics on Mild Gastrointestinal Disorders of Infants: A Single-Armed Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Study Formula
2.3. Outcome Measurements
2.4. Quality Control
2.5. Statistical Analysis
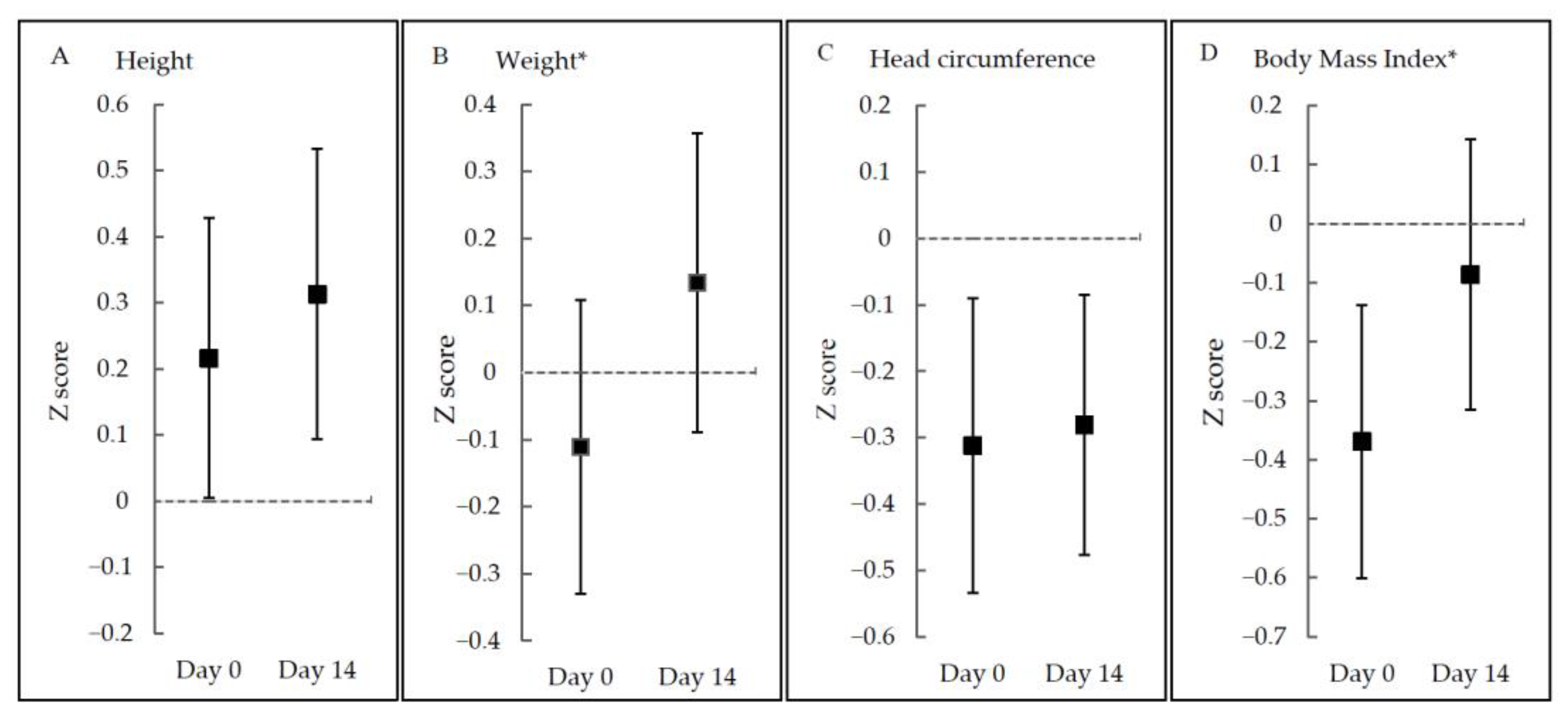
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Infante Pina, D.; Badia Llach, X.; Arino-Armengol, B.; Villegas Iglesias, V. Prevalence and dietetic management of mild gastroin-testinal disorders in milk-fed infants. World J. Gastroenterol. 2008, 14, 248–254. [Google Scholar] [CrossRef]
- Liem, O.; Harman, J.; Benninga, M.; Kelleher, K.; Mousa, H.; Di Lorenzo, C. Health utilization and cost impact of childhood con-stipation in the United States. J. Pediatr. 2009, 154, 258–262. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; ÇokuÐraþ, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and Health Outcomes of Func-tional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Xiao, L.P.; Yun, L.; Wang, X.Q.; Xu, C.D. Epidemiology of mild gastrointestinal disorders among infants and young children in Shanghai area. Zhonghua Er Ke Za Zhi 2009, 47, 917–921. (In Chinese) [Google Scholar]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Dis-orders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Miller-Loncar, C.; Bigsby, R.; High, P.; Wallach, M.; Lester, B. Infant colic and feeding difficulties. Arch. Dis. Child. 2004, 89, 908–912. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. 2013. Available online: https://apps.who.int/iris/handle/10665/84409 (accessed on 1 September 2021).
- World Health Organization. Breastfeeding. 2018. Available online: https://www.who.int/news-room/facts-in-pictures/detail/breastfeeding (accessed on 1 September 2021).
- Wang, Y.; Zhou, C. China should take more measures to raise its breastfeeding rate. Biosci. Trends 2019, 13, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Munasir, Z.; Hegar, B.; Kumarawati, D.; Suryawan, A.; Kadim, M.; Djais, J.T.; Basrowi, R.W.; Krisnamurti, D. A perspective on partially hydrolyzed protein infant formula in nonexclusively breastfed infants. Korean J. Pediatr. 2019, 62, 149–154. [Google Scholar] [CrossRef]
- Host, A. Frequency of cow’s milk allergy in childhood. Ann. Allergy Asthma Immunol. 2002, 89, 33–37. [Google Scholar] [CrossRef]
- Turco, R.; Russo, M.; Bruzzese, D.; Staiano, A. Efficacy of a partially hydrolysed formula, with reduced lactose content and with Lactobacillus reuteri DSM 17938 in infant colic: A double blind, randomised clinical trial. Clin. Nutr. 2021, 40, 412–419. [Google Scholar] [CrossRef]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef]
- Aloisio, I.; Prodam, F.; Giglione, E.; Cionci, N.B.; Solito, A.; Bellone, S.; Baffoni, L.; Mogna, L.; Pane, M.; Bona, G.; et al. Three-Month Feeding Integration With Bifidobacterium Strains Prevents Gastrointestinal Symptoms in Healthy Newborns. Front. Nutr. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihatsch, W.A.; Franz, A.R.; Högel, J.; Pohlandt, F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002, 110, 1199–1203. [Google Scholar] [CrossRef]
- Griffin, M.; Hansen, J.W. Can the elimination of lactose from formula improve feeding tolerance in premature infants? J. Pediatr. 1999, 135, 587–592. [Google Scholar] [CrossRef]
- Berseth, C.L.; Johnston, W.H.; Stolz, S.I.; Harris, C.L.; Mitmesser, S.H. Clinical Response to 2 Commonly Used Switch Formulas Occurs within 1 Day. Clin. Pediatr. 2008, 48, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.W.; Trabulsi, J.; Yao, M.; Bevans, K.B.; DeRusso, P.A. Validation of a Parent Report Questionnaire: The Infant Gastrointestinal Symptom Questionnaire. Clin. Pediatr. 2015, 54, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pados, B.F.; Basler, A. Gastrointestinal Symptoms in Healthy, Full-Term Infants Under 7 Months of Age. J. Pediatr. Nurs. 2020, 53, 1–5. [Google Scholar] [CrossRef]
- World Health Organization. The WHO Child Growth Standards. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 1 September 2021).
- Savino, F.; Cresi, F.; Maccario, S.; Cavallo, F.; Dalmasso, P.; Fanaro, S.; Oggero, R.; Vigi, V.; Silvestro, L. “Minor” feeding problems during the first months of life: Effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr. 2003, 91, 86–90. [Google Scholar] [CrossRef]
- Savino, F.; Palumeri, E.; Castagno, E.; Cresi, F.; Dalmasso, P.; Cavallo, F.; Oggero, R. Reduction of crying episodes owing to infantile colic: A randomized controlled study on the efficacy of a new infant formula. Eur. J. Clin. Nutr. 2006, 60, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.D.; Cabana, M.D. Partially Hydrolyzed 100% Whey Protein Infant Formula and Reduced Risk of Atopic Dermatitis: A Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 422–430. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Latiff, A.H.A.; Fleischer, D.M.; Gutiérrez-Castrellón, P.; Miqdady, M.; Smith, P.K.; von Berg, A.; Greenhawt, M.J. Partially hydrolyzed formula in non-exclusively breastfed infants: A systematic review and expert consensus. Nutrition 2019, 57, 268–274. [Google Scholar] [CrossRef]
- Scalabrin, D.; Harris, C.; Johnston, W.H.; Berseth, C.L. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: A 5-year follow-up. Eur. J. Nucl. Med. Mol. Imaging 2017, 176, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.R. Effects of prolonged exposure to partially hydrolyzed milk protein. J. Pediatr. 1992, 121, S90–S94. [Google Scholar] [CrossRef]
- Lambe, C.; Talbotec, C.; Kapel, N.; Piketty, M.; Goulet, O. 300.6: The REVE study, preliminary results. A Monocentric Single-arm study to characterize the long-term safety, efficacy, and pharmacodynamic of GLP-2 analog (Revestive®) in the management of short bowel syndrome pediatric patients on home-parenteral nutrition (HPN). Transplantation 2019, 103, S11. [Google Scholar] [CrossRef]
- Yang, E.M.; Lee, S.T.; Choi, H.J.; Cho, H.Y.; Lee, J.H.; Kang, H.G.; Park, Y.S.; Cheong, H.I.; Ha, I.-S. Tacrolimus for children with refractory nephrotic syndrome: A one-year prospective, multicenter, and open-label study of Tacrobell®, a generic formula. World J. Pediatr. 2015, 12, 60–65. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Cochrane, B.; Wopereis, H.; Loveridge, N. Specific prebiotics in a formula for infants with Phenylketonuria. Mol. Genet. Metab. 2011, 104, S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, J.; Nydegger, A.; Belli, D.; Furlano, R.I.; Yan, J.; Tanguy, J.; Pecquet, S.; Destaillats, F.; Egli, D.; Steenhout, P. Growth of Infants Fed Formula with Evolving Nutrition Composition: A Single-Arm Non-Inferiority Study. Nutrition 2017, 9, 219. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Number (%)/Mean ± SD |
|---|---|
| Sex | |
| Male | 39 (48.8) |
| Female | 41 (51.3) |
| Age (months) | 2.0 ± 1.5 |
| Delivery mode | |
| Vaginal delivery | 50 (62.5) |
| Cesarean delivery | 30 (37.5) |
| Birth weight (g) | 3266 ± 470 |
| Feeding practice | |
| Mixed feeding | 78 (97.5) |
| Exclusive formula feeding | 2 (2.5) |
| Maternal age (year) | 32.1 ± 5.3 |
| Paternal age (year) | 32.5 ± 5.8 |
| Maternal education * | |
| College or above | 46 (58.2) |
| Senior high school | 25 (31.6) |
| Junior high school or below | 8 (10.1) |
| Paternal education * | |
| College or above | 48 (60.8) |
| Senior high school | 23 (29.1) |
| Junior high school or below | 8 (10.1) |
| Domains (Score Range) | Day 0 (Baseline) Mean ± SD | Day 7 | Day 14 | |||
|---|---|---|---|---|---|---|
| Mean ± SD | MD (95% CI) vs. Day 0 | Mean ± SD | MD (95% CI) vs. Day 0 | MD (95% CI) vs. Day 7 | ||
| Vomiting (4 to 20) | 10.0 ± 2.9 | 7.9 ± 3.0 | −2.2 (−2.8, −1.5) * | 7.3 ± 2.9 | −2.7 (−3.4, −2.0) * | −0.5 (−1.2, 0.1) |
| Flatulence (3 to 15) | 7.7 ± 1.3 | 5.1 ± 1.9 | −2.6 (−3.0, −2.1) * | 4.8 ± 1.7 | −2.9 (−3.3, −2.5) * | −0.4 (−0.8, 0.1) |
| Crying (2 to 10) | 7.4 ± 2.7 | 6.4 ± 3.0 | −1.0 (−1.7, −0.3) * | 5.7 ± 2.7 | −1.8 (−2.4, −1.1) * | −0.8 (−1.4, −0.1) * |
| Fussiness (2 to 10) | 9.1 ± 2.1 | 7.6 ± 2.9 | −1.6 (−2.2, −1.0) * | 6.8 ± 2.8 | −2.4 (−3.0, −1.7) * | −0.8 (−1.2, −0.2) * |
| Stooling (2 to 10) | 4.1 ± 1.7 | 3.9 ± 2.0 | −0.2 (−0.7, 0.3) | 3.8 ± 1.9 | −0.3 (−0.8, 0.2) | −0.1 (−0.6, 0.4) |
| Total scores (13 to 65) | 36.0 ± 5.7 | 28.7 ± 7.4 | −7.3 (−9.0, −5.7) * | 26.5 ± 8.1 | −9.6 (−11.2, −7.9) * | −2.2 (−3.9, −0.5) * |
| Domain | Day 0 | Day 1 | Day 2 | Day 3 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD/Frequency (%) | Mean ± SD/Frequency (%) | MD (95% CI)/ OR (95% CI) | Mean ± SD/Frequency (%) | MD (95% CI)/ OR (95% CI) | Mean ± SD/Frequency (%) | MD (95% CI)/ OR (95% CI) | |
| Simplified IGSQ (score range) | |||||||
| Vomiting (1 to 5) | 3.6 ± 1.3 | 2.9 ± 1.3 | −0.7 (−0.9, −0.5) * | 2.7 ± 1.3 | −0.9 (−1.1, −0.7) * | 2.6 ± 1.3 | −1.0 (−1.2, −0.7) * |
| Flatulence (1 to 5) | 4.6 ± 0.8 | 2.8 ± 1.1 | −1.8 (−2.0, −1.6) * | 2.7 ± 1.1 | −1.9 (−2.1, −1.7) * | 2.7 ± 1.0 | −1.9 (−2.1, −1.7) * |
| Crying (1 to 5) | 2.4 ± 0.7 | 2.3 ± 1.1 | −0.1 (−0.3, 0.1) | 2.2 ± 1.0 | −0.2 (−0.4, −0.03) * | 2.0 ± 0.9 | −0.4 (−0.6, −0.2) * |
| Fussiness (1 to 5) | 3.3 ± 1.3 | 3.4 ± 1.3 | −0.03 (−0.2, 0.3) | 3.3 ± 1.4 | −0.04 (−0.4, 0.2) | ||
| Stool Characteristic | |||||||
| Pain or not | |||||||
| Without pain | 102 (82.9) | 1.0 (reference) | 90 (75.6) | 1.4 (0.5, 4.0) | 92 (74.2) | 1.0 (0.4, 2.8) | |
| With pain | 21 (17.1) | 29 (24.4) | 32 (25.8) | ||||
| Consistency | |||||||
| Watery stool | 16 (13.0) | 1.0 (reference) | 9 (7.6) | 0.99 (0.70, 1.40) | 10 (8.1) | 1.00 (0.71, 1.40) | |
| Runny stool | 52 (42.3) | 57 (47.9) | 62 (50.0) | ||||
| Mushy stool | 50 (40.7) | 50 (42.0) | 44 (35.5) | ||||
| Firm stool | 2 (1.6) | 3 (2.5) | 3 (2.4) | ||||
| Hard stool | 3 (2.4) | 0 (0.0) | 5 (4.0) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhou, Y.; Li, H.; Chen, Y.; Mu, Y.; Yuan, A.; Yang, Y.; Liu, J. The Effects of a Partially Hydrolyzed Formula with Low Lactose and Probiotics on Mild Gastrointestinal Disorders of Infants: A Single-Armed Clinical Trial. Nutrients 2021, 13, 3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103371
Huang Y, Zhou Y, Li H, Chen Y, Mu Y, Yuan A, Yang Y, Liu J. The Effects of a Partially Hydrolyzed Formula with Low Lactose and Probiotics on Mild Gastrointestinal Disorders of Infants: A Single-Armed Clinical Trial. Nutrients. 2021; 13(10):3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103371
Chicago/Turabian StyleHuang, Yongying, Yubo Zhou, Hongtian Li, Yipu Chen, Yingchao Mu, Anan Yuan, Yantao Yang, and Jianmeng Liu. 2021. "The Effects of a Partially Hydrolyzed Formula with Low Lactose and Probiotics on Mild Gastrointestinal Disorders of Infants: A Single-Armed Clinical Trial" Nutrients 13, no. 10: 3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103371





