Effects of Acute Heat and Cold Exposures at Rest or during Exercise on Subsequent Energy Intake: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Assessment of the Risk of Bias
2.6. Meta-Analysis Procedures and Statistical Approach
3. Results
3.1. Search Results
3.2. Study and Population Characteristics
3.3. Assessment of the Intervention: Temperature
3.4. Clothing Characteristics
3.5. Assessment of the Perceptive and Physiological Impact of Exposure to Heat or Cold
3.6. Characteristics of the Food and Exercise Interventions
3.7. Changes in Energy Intake
3.8. Risk of Bias across Studies
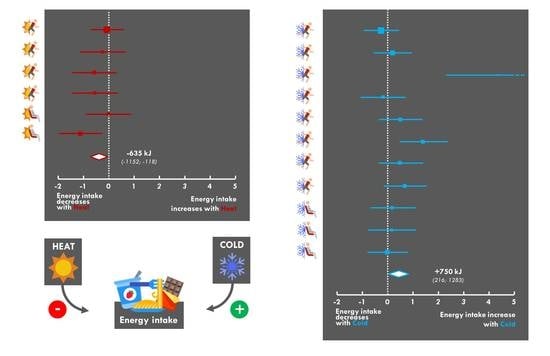
3.9. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasiakos, S.M. Nutritional Requirements for Sustaining Health and Performance during Exposure to Extreme Environments. Annu. Rev. Nutr. 2020, 40, 221–245. [Google Scholar] [CrossRef]
- Johnson, C.D.; Simonson, A.J.; Darnell, M.E.; DeLany, J.P.; Wohleber, M.F.; Connaboy, C. Energy expenditure and intake during Special Operations Forces field training in a jungle and glacial environment. Appl. Physiol. Nutr. Metab. 2017, 43, 381–386. [Google Scholar] [CrossRef]
- Saunders, P.U.; Garvican-Lewis, L.A.; Chapman, R.F.; Periard, J.D. Special Environments: Altitude and Heat. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Charlot, K.; Faure, C.; Antoine-Jonville, S. Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review. Nutrients 2017, 9, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelmach-Mardas, M.; Kleiser, C.; Uzhova, I.; Penalvo, J.L.; La Torre, G.; Palys, W.; Lojko, D.; Nimptsch, K.; Suwalska, A.; Linseisen, J.; et al. Seasonality of food groups and total energy intake: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 700–708. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. Effects of extreme environments on food intake in human subjects. Proc. Nutr. Soc. 1999, 58, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlot, K. Negative energy balance during military training: The role of contextual limitations. Appetite 2021, 164, 105263. [Google Scholar] [CrossRef]
- Tassone, E.C.; Baker, B.A. Body weight and body composition changes during military training and deployment involving the use of combat rations: A systematic literature review. Br. J. Nutr. 2017, 117, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Sato, T.; Nemoto, T.; Hasegawa, K.; Ida, T.; Kojima, M. A new action of peptide hormones for survival in a low-nutrient environment. Endocr. J. 2019, 66, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.E.; Carrigan, C.T.; Philip Karl, J.; Pasiakos, S.M.; Margolis, L.M. Threshold of Energy Deficit and Lower-Body Performance Declines in Military Personnel: A Meta-Regression. Sports Med. 2018, 48, 2169–2178. [Google Scholar] [CrossRef]
- Cian, C.; Koulmann, N.; Barraud, P.; Raphel, C.; Jimenez, C.; Melin, B. Influences of variations in body hydration on cognitive function: Effect of hyperhydration, heat stress, and exercise-induced dehydration. J. Psychophysiol. 2000, 14, 29–36. [Google Scholar] [CrossRef]
- Karl, J.P.; Thompson, L.A.; Niro, P.J.; Margolis, L.M.; McClung, J.P.; Cao, J.J.; Whigham, L.D.; Combs, G.F., Jr.; Young, A.J.; Lieberman, H.R.; et al. Transient decrements in mood during energy deficit are independent of dietary protein-to-carbohydrate ratio. Physiol. Behav. 2015, 139, 524–531. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Wardle, S.L.; Greeves, J.P. Energy Deficiency in Soldiers: The Risk of the Athlete Triad and Relative Energy Deficiency in Sport Syndromes in the Military. Front. Nutr. 2020, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.M.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [Green Version]
- White, L.J.; Dressendorfer, R.H.; Holland, E.; McCoy, S.C.; Ferguson, M.A. Increased caloric intake soon after exercise in cold water. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.R.; Blannin, A.K. Effects of exercise in the cold on Ghrelin, PYY, and food intake in overweight adults. Med. Sci. Sports Exerc. 2015, 47, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wasse, L.K.; King, J.A.; Stensel, D.J.; Sunderland, C. Effect of ambient temperature during acute aerobic exercise on short-term appetite, energy intake, and plasma acylated ghrelin in recreationally active males. Appl. Physiol. Nutr. Metab. 2013, 38, 905–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shorten, A.L.; Wallman, K.E.; Guelfi, K.J. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am. J. Clin. Nutr. 2009, 90, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski-Fruer, J.K.; Horsfall, R.N.; Cottrill, D.; Hough, J. Acute exposure to a hot ambient temperature reduces energy intake but does not affect gut hormones in men during rest. Br. J. Nutr. 2021, 125, 951–959. [Google Scholar] [CrossRef]
- Ahmed, M.; Mandic, I.; Lou, W.; Goodman, L.; Jacobs, I.; L’Abbe, M.R. Comparison of dietary intakes of Canadian Armed Forces personnel consuming field rations in acute hot, cold, and temperate conditions with standardized infantry activities. Mil. Med. Res. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Charlot, K.; Henri, S.; Hardy-Dessources, M.D.; Hue, O.; Antoine-Jonville, S. Effect of heat exposure and exercise on food intake regulation: A randomized crossover study in young healthy men. Metabolism 2016, 65, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, M.; Tan, C.Y.; Soeters, M.R.; Virtue, S.; Ambler, G.K.; Watson, L.P.; Murgatroyd, P.R.; Chatterjee, V.K.; Vidal-Puig, A. Mild cold effects on hunger, food intake, satiety and skin temperature in humans. Endocr. Connect. 2016, 5, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Li, K.; Wang, Y. The Effects of High-Temperature Weather on Human Sleep Quality and Appetite. Int. J. Environ. Res. Public Health 2019, 16, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, C.; Sasaki, H.; Tsuchiya, Y.; Goto, K. The influence of environmental temperature on appetite-related hormonal responses. J. Physiol. Anthropol. 2015, 34, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, T.L.; Zak, R.B.; Shute, R.J.; Heesch, M.W.S.; Dinan, N.E.; Bubak, M.P.; La Salle, D.T.; Slivka, D.R. Leptin, adiponectin, and ghrelin responses to endurance exercise in different ambient conditions. Temperature 2017, 4, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Westerterp-Plantenga, M.S.; van Marken Lichtenbelt, W.D.; Strobbe, H.; Schrauwen, P. Energy metabolism in humans at a lowered ambient temperature. Eur. J. Clin. Nutr. 2002, 56, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Kojima, C.; Kasai, N.; Kondo, C.; Ebi, K.; Goto, K. Post-Exercise Whole Body Cryotherapy (−140 degrees C) Increases Energy Intake in Athletes. Nutrients 2018, 10, 893. [Google Scholar] [CrossRef] [Green Version]
- Metz, L.; Isacco, L.; Beaulieu, K.; Fearnbach, S.N.; Pereira, B.; Thivel, D.; Duclos, M. Cold-Water Effects on Energy Balance in Healthy Women During Aqua-Cycling. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 236–243. [Google Scholar] [CrossRef]
- McInnis, K.; Haman, F.; Doucet, E. Humans in the cold: Regulating energy balance. Obes. Rev. 2020, 21, e12978. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Glossary of terms for thermal physiology. Second edition. Revised by The Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission). Pflug. Arch. Eur. J. Physiol. 1987, 410, 567–587.
- Kingma, B.; Frijns, A.; van Marken Lichtenbelt, W. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975–1985. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. 567. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressendorfer, R.H. Effect of internal body temperature on energy intake soon after aerobic exercise. Med. Sci. Sports Exerc. 1993, S42, 228. [Google Scholar] [CrossRef]
- Halse, R.E.; Wallman, K.E.; Guelfi, K.J. Postexercise Water Immersion Increases Short-Term Food Intake in Trained Men. Med. Sci. Sports Exerc. 2011, 43, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Burdon, C.A.; Johnson, N.A.; Chapman, P.G.; O’Connor, H.T. Influence of beverage temperature on palatability and fluid ingestion during endurance exercise: A systematic review. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Baskentli, S.; Block, L.; Morrin, M. The serving temperature effect: Food temperature, expected satiety, and complementary food purchases. Appetite 2021, 160, 105069. [Google Scholar] [CrossRef]
- Motoki, K.; Saito, T.; Nouchi, R.; Sugiura, M. Cross-Modal Correspondences Between Temperature and Taste Attributes. Front. Psychol. 2020, 11, 571852. [Google Scholar] [CrossRef]
- Brearley, M.B. Should Workers Avoid Consumption of Chilled Fluids in a Hot and Humid Climate? Saf. Health Work 2017, 8, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bryant, E.; Blundell, J.E.; King, N.A. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol. Behav. 2009, 97, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Chapelot, D. Energy compensation after an aerobic exercise session in high-fat/low-fit and low-fat/high-fit young male subjects. Br. J. Nutr. 2013, 110, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.A.; Wasse, L.K.; Stensel, D.J. Acute exercise increases feeding latency in healthy normal weight young males but does not alter energy intake. Appetite 2013, 61, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malgoyre, A.; Siracusa, J.; Tardo-Dino, P.E.; Garcia-Vicencio, S.; Koulmann, N.; Charlot, K. A basal heat stress test to detect military operational readiness after a 14-day operational heat acclimatization period. Temperature 2020, in press. [Google Scholar] [CrossRef]
- Alkemade, P.; Gerrett, N.; Eijsvogels, T.M.H.; Daanen, H.A.M. Individual characteristics associated with the magnitude of heat acclimation adaptations. Eur. J. Appl. Physiol. 2021, 121, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Brychta, R.J.; Chen, K.Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 2017, 71, 345–352. [Google Scholar] [CrossRef]
- Ivanova, Y.M.; Pallubinsky, H.; Kramer, R.; van Marken Lichtenbelt, W. The influence of a moderate temperature drift on thermal physiology and perception. Physiol. Behav. 2021, 229, 113257. [Google Scholar] [CrossRef]
- Cheung, S.S. Interconnections between thermal perception and exercise capacity in the heat. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. 3), 53–59. [Google Scholar] [CrossRef]
- Ross, R.; Soni, S.; Houle, S.A. Negative Energy Balance Induced by Exercise or Diet: Effects on Visceral Adipose Tissue and Liver Fat. Nutrients 2020, 12, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, M.E.; McNeill, G.; Brockway, J.M.; Smith, J.S. The effect of environmental temperature and humidity on 24 h energy expenditure in men. Br. J. Nutr. 1992, 68, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocobock, C. Human energy expenditure, allocation, and interactions in natural temperate, hot, and cold environments. Am. J. Phys. Anthropol. 2016, 161, 667–675. [Google Scholar] [CrossRef]
- Tharion, W.J.; Lieberman, H.R.; Montain, S.J.; Young, A.J.; Baker-Fulco, C.J.; Delany, J.P.; Hoyt, R.W. Energy requirements of military personnel. Appetite 2005, 44, 47–65. [Google Scholar] [CrossRef]
- Tanskanen, M.M.; Westerterp, K.R.; Uusitalo, A.L.; Atalay, M.; Hakkinen, K.; Kinnunen, H.O.; Kyrolainen, H. Effects of easy-to-use protein-rich energy bar on energy balance, physical activity and performance during 8 days of sustained physical exertion. PLoS ONE 2012, 7, e47771. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb. Clin. Neurol. 2018, 156, 153–173. [Google Scholar] [CrossRef]
- Layden, J.D.; Patterson, M.J.; Nimmo, M.A. Effects of reduced ambient temperature on fat utilization during submaximal exercise. Med. Sci. Sports Exerc. 2002, 34, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, D.D.; Rintamaki, H.; Gagnon, S.S.; Cheung, S.S.; Herzig, K.H.; Porvari, K.; Kyrolainen, H. Cold exposure enhances fat utilization but not non-esterified fatty acids, glycerol or catecholamines availability during submaximal walking and running. Front. Physiol. 2013, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [Green Version]
- Stensel, D. Exercise, Appetite and Appetite-Regulating Hormones: Implications for Food Intake and Weight Control. Ann. Nutr. Metab. 2010, 57, 36–42. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gibbons, C.; Beaulieu, K.; Casanova, N.; Duarte, C.; Finlayson, G.; Stubbs, R.J.; Hopkins, M. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol. Behav. 2020, 219, 112846. [Google Scholar] [CrossRef] [PubMed]
- Chapelot, D.; Charlot, K. Physiology of energy homeostasis: Models, actors, challenges and the glucoadipostatic loop. Metabolism 2019, 92, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Mandic, I.; Ahmed, M.; Rhind, S.; Goodman, L.; L’Abbe, M.; Jacobs, I. The effects of exercise and ambient temperature on dietary intake, appetite sensation, and appetite regulating hormone concentrations. Nutr. Metab. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeyl, A.; Stocks, J.M.; Taylor, N.A.S.; Jenkins, A.B. Interactions between temperature and human leptin physiology in vivo and in vitro. Eur. J. Appl. Physiol. 2004, 92, 571–578. [Google Scholar] [CrossRef]
- Tomasik, P.J.; Sztefko, K.; Pizon, M. The effect of short-term cold and hot exposure on total plasma ghrelin concentrations in humans. Horm. Metab. Res. Horm. Stoffwechs. Horm. Métab. 2005, 37, 189–190. [Google Scholar] [CrossRef]
- Suwanapaporn, P.; Chaiyabutr, N.; Thammacharoen, S. A low degree of high ambient temperature decreased food intake and activated median preoptic and arcuate nuclei. Physiol. Behav. 2017, 181, 16–22. [Google Scholar] [CrossRef]
- Bohler, M.; Gilbert, E.R.; Cline, M.A. Reduced food intake during exposure to high ambient temperatures is associated with molecular changes in the nucleus of the hippocampal commissure and the paraventricular and arcuate hypothalamic nuclei. Gen. Comp. Endocrinol. 2020, 298, 113576. [Google Scholar] [CrossRef]
- Zhao, N.; Mu, L.; Chang, X.; Zhu, L.; Geng, Y.; Li, G. Effects of varying intensities of heat stress on neuropeptide Y and proopiomelanocortin mRNA expression in rats. Biomed. Rep. 2020, 13, 39. [Google Scholar] [CrossRef]
- Morton, G.J.; Schwartz, M.W. The NPY/AgRP neuron and energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001, 25 (Suppl. 5), S56–S62. [Google Scholar] [CrossRef]
- Bing, C.; Pickavance, L.; Wang, Q.; Frankish, H.; Trayhurn, P.; Williams, G. Role of hypothalamic neuropeptide Y neurons in the defective thermogenic response to acute cold exposure in fatty Zucker rats. Neuroscience 1997, 80, 277–284. [Google Scholar] [CrossRef]
- Xing, X.; Liu, S.; Liu, X.Y.; Yang, M.; Wang, D.H. Cold exposure increased hypothalamic orexigenic neuropeptides but not food intake in fattening Daurian ground squirrels. Zoology 2020, 143, 125834. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Kilpatrick, A.P.; Trayhurn, P.; Williams, G. Widespread increases in regional hypothalamic neuropeptide Y levels in acute cold-exposed rats. Neuroscience 1993, 54, 127–132. [Google Scholar] [CrossRef]
- Lau, J.; Shi, Y.C.; Herzog, H. Temperature dependence of the control of energy homeostasis requires CART signaling. Neuropeptides 2016, 59, 97–109. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, H.K.; Shin, M.W.; Kim, S.J.; Noh, S.Y.; Shin, J.; Yu, W.S. Short-term cold exposure may cause a local decrease of neuropeptide Y in the rat hypothalamus. Mol. Cells 2007, 23, 88–93. [Google Scholar]
- Deem, J.D.; Faber, C.L.; Pedersen, C.; Phan, B.A.; Larsen, S.A.; Ogimoto, K.; Nelson, J.T.; Damian, V.; Tran, M.A.; Palmiter, R.D.; et al. Cold-induced hyperphagia requires AgRP neuron activation in mice. eLife 2020, 9, e58764. [Google Scholar] [CrossRef]
- Faure, C.; Charlot, K.; Henri, S.; Hardy-Dessources, M.D.; Hue, O.; Antoine-Jonville, S. Impaired glucose tolerance after brief heat exposure: A randomized crossover study in healthy young men. Clin. Sci. 2016, 130, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Naperalsky, M.; Ruby, B.; Slivka, D. Environmental temperature and glycogen resynthesis. Int. J. Sports Med. 2010, 31, 561–566. [Google Scholar] [CrossRef]
- Dumke, C.L.; Slivka, D.R.; Cuddy, J.S.; Hailes, W.S.; Rose, S.M.; Ruby, B.C. The Effect of Environmental Temperature on Glucose and Insulin after an Oral Glucose Tolerance Test in Healthy Young Men. Wilderness Environ. Med. 2015, 26, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Antoine-Jonville, S.; El Khoury, D.; Faure, C.; Charlot, K.; Hue, O.; Hardy-Dessources, M.D. Metabolic response to oral glucose tolerance test performed in neutral and warm environmental temperature. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthemic Oncol. N. Am. Hyperth. Group 2019, 36, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Angus, D.; Howlett, K.; Conus, N.M.; Febbraio, M. Effect of heat stress on glucose kinetics during exercise. J. Appl. Physiol. 1996, 81, 1594–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbraio, M.A.; Lambert, D.L.; Starkie, R.L.; Proietto, J.; Hargreaves, M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J. Appl. Physiol. 1998, 84, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Melanson, K.J.; Westerterp-Plantenga, M.S.; Saris, W.H.; Smith, F.J.; Campfield, L.A. Blood glucose patterns and appetite in time-blinded humans: Carbohydrate versus fat. Am. J. Physiol. 1999, 277, R337–R345. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti-de-Albuquerque, J.P.; Kincheski, G.C.; Louzada, R.A.; Galina, A.; Pierucci, A.; Carvalho, D.P. Intense physical exercise potentiates glucose inhibitory effect over food intake of male Wistar rats. Exp. Physiol. 2018, 103, 1076–1086. [Google Scholar] [CrossRef]
- Langhans, W.; Grossmann, F.; Geary, N. Intrameal hepatic-portal infusion of glucose reduces spontaneous meal size in rats. Physiol. Behav. 2001, 73, 499–507. [Google Scholar] [CrossRef]
- Adachi, A.; Shimizu, N.; Oomura, Y.; Kobashi, M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci. Lett. 1984, 46, 215–218. [Google Scholar] [CrossRef]
- Niijima, A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J. Auton. Nerv. Syst. 1983, 9, 207–220. [Google Scholar] [CrossRef]
- Lavin, J.H.; Wittert, G.; Sun, W.M.; Horowitz, M.; Morley, J.E.; Read, N.W. Appetite regulation by carbohydrate: Role of blood glucose and gastrointestinal hormones. Am. J. Physiol. 1996, 271, E209–E214. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Hentges, S.T.; Dunn-Meynell, A.A.; Levin, B.E.; Wang, W.; Routh, V.H. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 2004, 53, 1959–1965. [Google Scholar] [CrossRef] [Green Version]
- Oomura, Y.; Ooyama, H.; Sugimori, M.; Nakamura, T.; Yamada, Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature 1974, 247, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Oomura, Y.; Yoshimatsu, H. Neural network of glucose monitoring system. J. Auton. Nerv. Syst. 1984, 10, 359–372. [Google Scholar] [CrossRef]
- Routh, V.H. Glucose-sensing neurons: Are they physiologically relevant? Physiol. Behav. 2002, 76, 403–413. [Google Scholar] [CrossRef]
- Burdakov, D.; Gonzalez, J.A. Physiological functions of glucose-inhibited neurones. Acta Physiol. 2009, 195, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Bain, A.R.; Morrison, S.A.; Ainslie, P.N. Cerebral oxygenation and hyperthermia. Front. Physiol. 2014, 5, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reminy, K.; Hue, O.; Antoine-Jonville, S. Effect of warm environment on the skin blood flow response to food intake. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2020, 37, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring food preference and reward: Application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J. The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef]
- Thackray, A.E.; Willis, S.A.; Sherry, A.P.; Clayton, D.J.; Broom, D.R.; Demashkieh, M.; Sargeant, J.A.; James, L.J.; Finlayson, G.; Stensel, D.J.; et al. An acute bout of swimming increases post-exercise energy intake in young healthy men and women. Appetite 2020, 154, 104785. [Google Scholar] [CrossRef]
- Beaulieu, K.; Hopkins, M.; Gibbons, C.; Oustric, P.; Caudwell, P.; Blundell, J.; Finlayson, G. Exercise Training Reduces Reward for High-Fat Food in Adults with Overweight/Obesity. Med. Sci. Sports Exerc. 2020, 52, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Charlot, K.; Chapelot, D.; Siracusa, J.; Lavoue, C.; Colin, P.; Oustric, P.; Thivel, D.; Finlayson, G.; Bourrilhon, C. An augmented food strategy leads to complete energy compensation during a 15-day military training expedition in the cold. Physiol. Rep. 2021, 9, e14591. [Google Scholar] [CrossRef]
- Aeberli, I.; Erb, A.; Spliethoff, K.; Meier, D.; Gotze, O.; Fruhauf, H.; Fox, M.; Finlayson, G.S.; Gassmann, M.; Berneis, K.; et al. Disturbed eating at high altitude: Influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 2013, 52, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R.; Meijer, E.P.; Rubbens, M.; Robach, P.; Richalet, J.P. Operation Everest III: Energy and water balance. Pflug. Arch. Eur. J. Physiol. 2000, 439, 483–488. [Google Scholar] [CrossRef]
- Burstein, R.; Coward, A.W.; Askew, W.E.; Carmel, K.; Irving, C.; Shpilberg, O.; Moran, D.; Pikarsky, A.; Ginot, G.; Sawyer, M.; et al. Energy expenditure variations in soldiers performing military activities under cold and hot climate conditions. Mil. Med. 1996, 161, 750–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whybrow, S.; Hughes, D.A.; Ritz, P.; Johnstone, A.M.; Horgan, G.W.; King, N.; Blundell, J.E.; Stubbs, R.J. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. Br. J. Nutr. 2008, 100, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Paravidino, V.B.; Mediano, M.F.F.; Crochemore-Silva, I.; da Cruz, V.L.; Antunes, M.M.L.; Beaulieu, K.; Gibbons, C.; Finlayson, G.; Blundell, J.E.; Sichieri, R. The compensatory effect of exercise on physical activity and energy intake in young men with overweight: The EFECT randomised controlled trial. Physiol. Behav. 2021, 229, 113249. [Google Scholar] [CrossRef]
- Rogers, M.A.; Appaneal, R.N.; Hughes, D.; Vlahovich, N.; Waddington, G.; Burke, L.M.; Drew, M. Prevalence of impaired physiological function consistent with Relative Energy Deficiency in Sport (RED-S): An Australian elite and pre-elite cohort. Br. J. Sports Med. 2021, 55, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mullie, P.; Clarys, P.; De Bry, W.; Geeraerts, P. Energy availability and nutrition during a Special Force Qualification Course (Q-Course). J. R. Army Med. Corps 2018, 165, 325–329. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millet, J.; Siracusa, J.; Tardo-Dino, P.-E.; Thivel, D.; Koulmann, N.; Malgoyre, A.; Charlot, K. Effects of Acute Heat and Cold Exposures at Rest or during Exercise on Subsequent Energy Intake: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3424. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103424
Millet J, Siracusa J, Tardo-Dino P-E, Thivel D, Koulmann N, Malgoyre A, Charlot K. Effects of Acute Heat and Cold Exposures at Rest or during Exercise on Subsequent Energy Intake: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(10):3424. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103424
Chicago/Turabian StyleMillet, Juliette, Julien Siracusa, Pierre-Emmanuel Tardo-Dino, David Thivel, Nathalie Koulmann, Alexandra Malgoyre, and Keyne Charlot. 2021. "Effects of Acute Heat and Cold Exposures at Rest or during Exercise on Subsequent Energy Intake: A Systematic Review and Meta-Analysis" Nutrients 13, no. 10: 3424. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103424