Obesity, Sodium Homeostasis, and Arterial Hypertension in Children and Adolescents
Abstract
:1. Introduction
2. Recommendations vs. Real Salt Intake in a Pediatric Population
3. Salt Consumption and a Risk of Arterial Hypertension in Children with Obesity
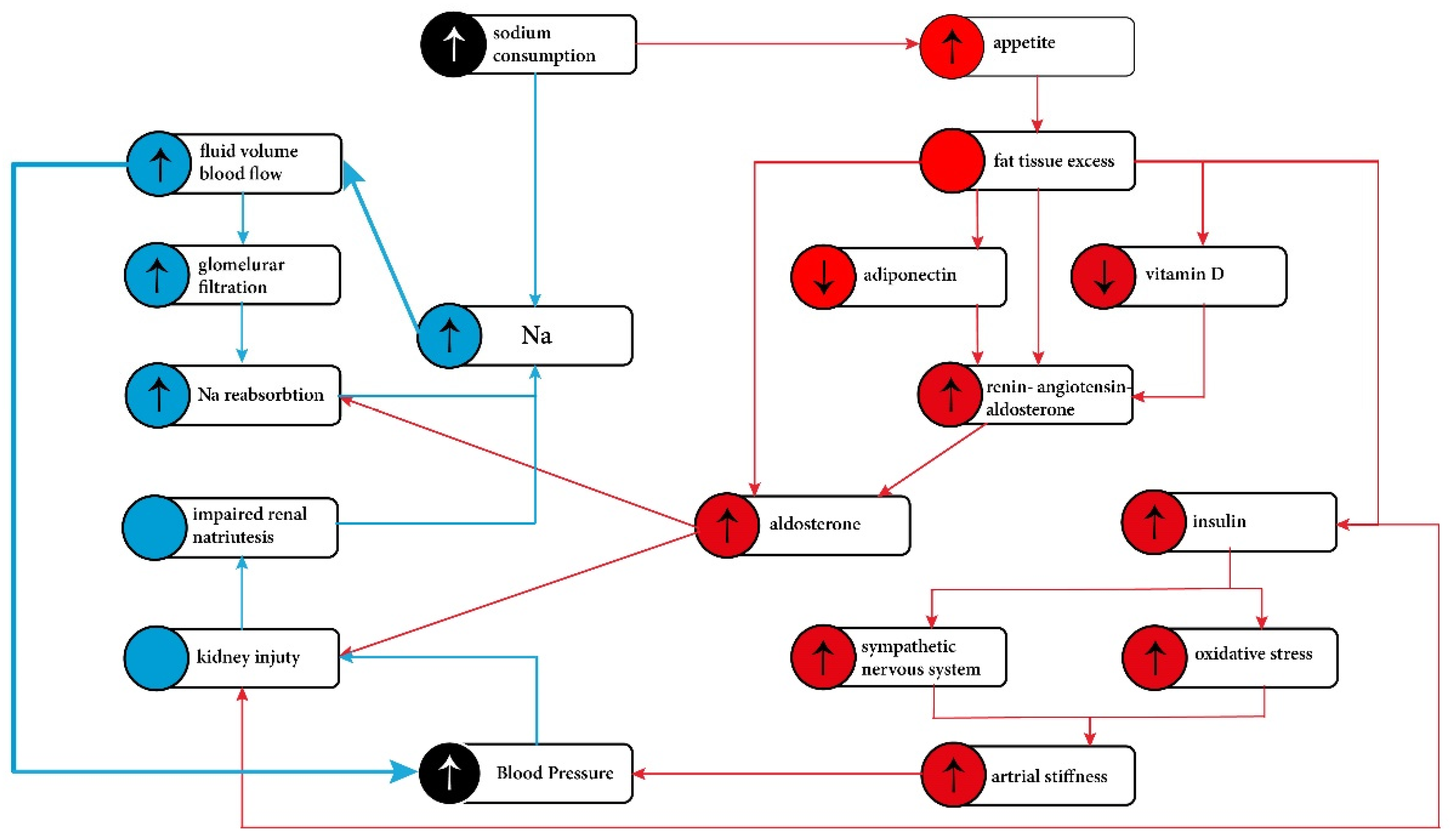
4. Proposed Mechanisms of Sodium Induced Hypertension in Obesity
4.1. Increased Extracellular Fluid Volume and Impaired Sodium Excretion
4.2. Mineralocorticoids/Mineralocorticoid Receptor
4.3. Hyperinsulinemia/Insulin Resistance
5. Directions in the Development of Salt Intake Recommendations, including Individualization in Patients with Obesity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 1289 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Kollias, A.; Skliros, E.; Stergiou, G.S.; Leotsakos, N.; Saridi, M.; Garifallos, D. Obesity and associated cardiovascular risk factors among schoolchildren in Greece: A cross-sectional study and review of the literature. J. Pediatr. Endocrinol. Metab. 2011, 24, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Sorof, J.M.; Lai, D.; Turner, J.; Poffenbarger, T.; Portman, R.J. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004, 113, 475–482. [Google Scholar] [CrossRef]
- Aparicio, A.; Rodríguez-Rodríguez, E.; Cuadrado-Soto, E.; Navia, B.; López-Sobaler, A.M.; Ortega, R.M. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7–11 years. Eur. J. Nutr. 2017, 56, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ambard, L.; Beaujard, E. Causes de l’hypertension artérielle. Arch. Gén. Méd. 1904, 81, 520–533. [Google Scholar]
- Vague, J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am. J. Clin. Nutr. 1956, 4, 20–34. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion Dietary reference values for sodium. EFSA J. 2019, 17, 5778. [Google Scholar]
- Huybrechts, I.; De Henauw, S. Energy and nutrient intakes by pre-school children in Flanders-Belgium. Br. J. Nutr. 2007, 98, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glynn, L.; Emmett, P.; Rogers, I.; ALSPAC Study Team. Food and nutrient intakes of a population sample of 7-year-old children in the south-west of England in 1999–2000—what difference does gender make? J. Hum. Nutr. Diet. 2005, 18, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Artalejo, F.; Garcés, C.; Gorgojo, L.; López García, E.; Martín-Moreno, J.M.; Benavente, M.; del Barrio, J.L.; Rubio, R.; Ortega, H.; Fernández, O.; et al. Dietary patterns among children aged 6–7 y in four Spanish cities with widely differing cardiovascular mortality. Eur. J. Clin. Nutr. 2002, 56, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Smpokos, E.A.; Linardakis, M.; Papadaki, A.; Theodorou, A.S.; Havenetidis, K.; Kafatos, A. Differences in energy and nutrient-intake among Greek children between 1992/93 and 2006/07. J. Hum. Nutr. Diet. 2014, 27, 230–238. [Google Scholar] [CrossRef]
- Herra-Majem, L.; Ribas-Barba, L.; Pérez-Rodrigo, C.; Bartrina, J.A. Nutrient adequacy in Spanish children and adolescents. Br. J. Nutr. 2006, 96, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.N.; Colantonio, L.D.; Howard, G.; Safford, M.M.; Banach, M.; Reynolds, K.; Cushman, M.; Muntner, P. Healthy lifestyle factors and incident heart disease and mortality in candidates for primary prevention with statin therapy. Int. J. Cardiol. 2016, 207, 196–202. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. Clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Couch, S.C.; Saelens, B.E.; Khoury, P.R.; Dart, K.B.; Hinn, K.; Mitsnefes, M.M.; Daniels, S.R.; Urbina, E.M. Dietary Approaches to Stop Hypertension Dietary Intervention Improves Blood Pressure and Vascular Health in Youth With Elevated Blood Pressure. Hypertension 2021, 77, 241–251. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children. 2012. Available online: http://www.who.int/nutrition/publications/guidelines/sodium_intake/en/ (accessed on 17 September 2021).
- Brouillard, A.M.; Deych, E.; Canter, C.; Rich, M.W. MD Trends in Sodium Intake in Children and Adolescents in the US and the Impact of US Department of Agriculture Guidelines: NHANES 2003–2016. J. Pediatr. 2020, 225, 117–123. [Google Scholar] [CrossRef]
- Krzysztoszek, J.; Kleka, P.; Laudańska-Krzemińska, I. Assessment of selected nutrient intake by Polish preschool children compared to dietary recommendations: A meta-analysis. Arch. Med. Sci. 2020, 16, 635–647. [Google Scholar] [CrossRef]
- Gonçalves, C.; Abreu, S.; Padrão, P.; Pinho, O.; Graça, P.; Breda, J.; Santos, R.; Moreira, P. Sodium and potassium urinary excretion and dietary intake: A cross-sectional analysis in adolescents. Food Nutr. Res. 2016, 60, 29442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneton, P.; Lafay, L.; Tard, A.; Dufour, A.; Ireland, J.; Menard, J.; Volatier, J.L. Dietary sources and correlates of sodium and potassium intakes in the French general population. Eur. J. Clin. Nutr. 2009, 63, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, C.A.; Riddell, L.J.; Campbell, K.J. Dietary salt intake, sugar sweetened beverage consumption, and obesity risk. Pediatrics 2013, 131, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Campanozzi, A.; Avallone, S.; Barbato, A.; Iacone, R.; Russo, O.; De Filippo, G.; D’Angelo, G.; Pensabene, L.; Malamisura, B.; Cecere, G.; et al. High sodium and low potassium intake among Italian children: Relationship with age, body mass and blood pressure. PLoS ONE 2015, 10, e0121183. [Google Scholar] [CrossRef]
- Maldonado-Martin, A.; Garcia-Matarin, L.; Gil-Extremera, B.; Avivar-Oyonarte, C.; Garcia-Granados, M.E.; Gil-Garcia, F.; Latorre-Hernández, J.; Miró-Gutiérrez, J.; Soria-Bonilla, A.; Vergara-Martín, J.; et al. Blood pressure and urinary excretion of electrolytes in Spanish schoolchildren. J. Hum. Hypertens. 2002, 1, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Marrero, N.M.; He, F.J.; Whincup, P.; Macgregor, G.A. Salt intake of children and adolescents in South London: Consumption levels and dietary sources. Hypertension 2014, 63, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Krupp, D.; Remer, T. Salt, fruit and vegetable consumption and blood pressure development: A longitudinal investigation in healthy children. Br. J. Nutr. 2014, 111, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; Dagenais, G.; Lear, S.; McQueen, M.; Diaz, R.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet 2016, 388, 465–475. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; Abe, M.; et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl saltsensitive rats. Hypertension 2002, 40, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Prada, P.O.; Coelho, M.S.; Zecchin, H.G.; Dolnikoff, M.S.; Gasparetti, A.L.; Furukawa, L.N.; Saad, M.J.; Heimann, J.C. Low salt intake modulates insulin signaling, JNK activity and IRS-1ser307 phosphorylation in rat tissues. J. Endocrinol. 2005, 185, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C.; Weinberger, M.H. Heterogenous responses to changes in diet ary salt intake: The salt-sensitivity paradigm. Am. J. Clin. Nutr. 1997, 65 (Suppl. 2), 612S–617S. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Rocchini, A.P.; Key, J.; Bondie, D. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N. Engl. J. Med. 1989, 321, 580–585. [Google Scholar] [CrossRef]
- Leyvraz, M.; Chatelan, A.; da Costa, B.R.; Taffé, P.; Paradis, G.; Bovet, P.; Bochud, M.; Chiolero, A. Sodium intake and blood pressure in children and adolescents: A systematic review and meta-analysis of experimental and observational studies. Int. J. Epidemiol. 2018, 47, 1796–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia-Costa, L.; Cosme, D.; Nogueira-Silva, L.; Morato, M.; Sousa, T.; Moura, C.; Mota, C.; Guerra, A.; Albino-Teixeira, A.; Areias, J.C.; et al. Gender and obesity modify the impact of salt intake on blood pressure in children. Pediatr. Nephrol. 2016, 31, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhang, Z.; Kuklina, E.V.; Fang, J.; Ayala, C.; Hong, Y.; Loustalot, F.; Dai, S.; Gunn, J.P.; Tian, N.; et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics 2012, 130, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gu, D.; Huang, J.; Rao, D.C.; Jaquish, C.E.; Hixson, J.E.; Chen, C.-S.; Chen, J.; Lu, F.; Hu, D.; et al. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: A dietary intervention study. Lancet 2009, 373, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef]
- Hall, J.E. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am. J. Hypertens. 1997, 10, 49s–55s. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Fujita, T. The Role of Aldosterone in Obesity-Related Hypertension. Am. J. Hypertens. 2016, 29, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feraco, A.; Marzolla, V.; Scuteri, A.; Armani, A.; Caprio, M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol. Metab. 2020, 31, 205–217. [Google Scholar] [CrossRef]
- De Mello, W.; Frohlich, E. Clinical perspectives and fundamental aspects of local cardiovascular and renal Renin-Angiotensin systems. Front. Endocrinol. 2014, 5, 16–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudenbostel, T.; Li, P.; Calhoun, D.A. Paradoxical Increase of 24-Hour Urinary Aldosterone Levels in Obese Patients with Resistant Hypertension on a High Salt Diet. Am. J. Hypertens. 2021, 34, 600–608. [Google Scholar] [CrossRef]
- Briones, A.; Cat, A.N.; Callera, G.; Yogi, A.; Burger, D.; He, Y.; Correa, J.W.; Gagnon, A.; Gomez-Sanchez, C.; Gomez-Sanchez, E.; et al. Adipocytes Produce Aldosterone Through Calcineurin-Dependent Signaling Pathways: Implications in Diabetes Mellitus–Associated Obesity and Vascular Dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Cat, A.N.; Friederich-Persson, M.; White, A.D.; Touyz, R. Adipocytes, aldosterone and obesity-related hypertension. J. Mol. Endocrinol. 2016, 57, F7–F21. [Google Scholar]
- Castrop, H.; Höcherl, K.; Kurtz, A.; Schweda, F.; Todorov, V.; Wagner, C. Physiology of kidney renin. Physiol. Rev. 2010, 90, 607–673. [Google Scholar] [CrossRef]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Adiponectin and hypertension. Am. J. Hypertens. 2011, 24, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Cheng, C.; Wang, Y.; Sun, H.; Yu, S.; Xue, Y.; Liu, Y.; Li, W.; Li, X. Effect of Vitamin D on Blood Pressure and Hypertension in the General Population: An Update Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Prev. Chronic Dis. 2020, 17, 190307. [Google Scholar] [CrossRef] [Green Version]
- Pelczyńska, M.; Grzelak, T.; Walczak, M.; Czyżewska, K. Hypovitaminosis D and adipose tissue—cause and effect relationships in obesity. Ann. Agric. Environ. Med. 2016, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Giovannucci, E.; Holmes, M.D.; Bischoff-Ferrari, H.A.; Tworoger, S.S.; Willett, W.C.; Curhan, G.C. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007, 49, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Nie, X.L.; Wu, S.; Cai, J. Vitamin D and hypertension: Prospective study and meta-analysis. PLoS ONE 2017, 12, e0174298. [Google Scholar] [CrossRef] [Green Version]
- Cuffee, Y.L.; Wang, M.; Geyer, N.R.; Saxena, S.; Akuley, S.; Jones, L.; Wilson, R.T. Vitamin D and family history of hypertension in relation to hypertension status among college students. J. Hum. Hypertens. 2021. [Google Scholar] [CrossRef] [PubMed]
- Alaagib, N.; Sukkar, M.; Kardash, M. The Effects of Salt and Glucose Intake on Angiotensin II and Aldosterone in Obese and Nonobese Patients with Essential Hypertension. Int. J. Hypertens. 2020, 2020, 6017105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; He, F.J.; MacGregor, G.A. High salt intake: Independent risk factor for obesity? Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef]
- Reinehr, T.; Andler, W. Cortisol and its relation to insulin resistance before and after weight loss in obese children. Horm. Res. 2004, 62, 107–112. [Google Scholar] [CrossRef]
- Castro, M.; Elias, P.C.L.; Martinelli, C.E., Jr.; Antonini, S.R.R.; Santiago, L.; Moreira, A.C. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz. J. Med. Biol. Res. 2000, 33, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawachi, H.; Fujita, T. Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension 2007, 49, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Whaley-Connell, A.; Sowers, J.R. Insulin Resistance in Kidney Disease: Is There a Distinct Role Separate from That of Diabetes or Obesity? Cardiorenal Med. 2017, 8, 41–49. [Google Scholar] [CrossRef]
- He, F.J.; Markandu, N.D.; Sagnella, G.A.; MacGregor, G.A. Effect of salt intake on renal excretion of water in humans. Hypertension 2001, 38, 317–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [Green Version]
- Lohmeier, T.E.; Iliescu, R.; Liu, B.; Henegar, J.R.; Maric-Bilkan, C.; Irwin, E.D. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012, 59, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggio, R.; Gutierrez, L.; Matta, M.G.; Elorriaga, N.; Irazola, V.; Rubinstein, A. Daily sodium consumption and CVD mortality in the general population: Systematic review and meta-analysis of prospective studies. Public Health Nutr. 2015, 18, 695–704. [Google Scholar] [CrossRef]
- World Health Organization. Vienna declaration on nutrition and noncommunicable diseases in the context of health 2020. In Proceedings of the WHO European Ministerial Conference on Nutrition and Noncommunicable Diseases in the Context of Health 2020, Vienna, Austria, 4–5 July 2013. [Google Scholar]
- Sharma, S.; McFann, K.; Chonchol, M.; Kendrick, J. Dietary sodium and potassium intake is not associated with elevated blood pressure in US adults with no prior history of hypertension. J. Clin. Hypertens. 2014, 16, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Umesawa, M.; Iso, H.; Date, C.; Yamamoto, A.; Toyoshima, H.; Watanabe, Y.; Kikuchi, S.; Koizumi, A.; Kondo, T.; Inaba, Y.; et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: The Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am. J. Clin. Nutr. 2008, 88, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Geleijnse, J.M.; Grobbee, D.E.; Hofman, A. Sodium and potassium intake and blood pressure change in childhood. BMJ 1990, 300, 899–902. [Google Scholar] [CrossRef] [Green Version]
- Adrogué, H.J.; Madias, N.E. Sodium and potassium in the pathogenesis of hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Byon, C.H.; Yang, Y.; Bradley, W.E.; Dell’Italia, L.J.; Sanders, P.W.; Agarwal, A.; Wu, H.; Chen, Y. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight 2017, 2, e94920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, N.R.; Obarzanek, E.; Cutler, J.A.; Buring, J.E.; Rexrode, K.M.; Kumanytka, S.K. Joint effects of sodium and potassium intak on subsequent cardiovascular disease. Arch. Intern. Med. 2009, 169, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelton, P.; He, J.; Culter, J.; Brancati, F.; Appel, L.; Follmann, D.; Klag, M. Effects of oral potassium on blood pressure: Meta-analysis of randomized controlled clinical trials. JAMA 1997, 277, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper From the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Ardissino, G.; Perrone, M.; Ghiglia, S.; Salice, P.; Tel, F.; Dardi, E.; Bollani, T.; Mezzopane, A.; Capone, V.; Ardissino, M.; et al. Fluid Intake and Blood Pressure in Children. The Spa Project. Available online: https://ssrn.com/abstract=3781634 (accessed on 10 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Kozioł-Kozakowska, A. Obesity, Sodium Homeostasis, and Arterial Hypertension in Children and Adolescents. Nutrients 2021, 13, 4032. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13114032
Wójcik M, Kozioł-Kozakowska A. Obesity, Sodium Homeostasis, and Arterial Hypertension in Children and Adolescents. Nutrients. 2021; 13(11):4032. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13114032
Chicago/Turabian StyleWójcik, Małgorzata, and Agnieszka Kozioł-Kozakowska. 2021. "Obesity, Sodium Homeostasis, and Arterial Hypertension in Children and Adolescents" Nutrients 13, no. 11: 4032. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13114032





