DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring
Abstract
:1. Introduction
2. Methods
2.1. Females Recruited for Breading as Mothers for Offspring Study Production
2.2. Measurement of Milk Composition
2.3. Milk Production and Pup Intake of Different Milk Components
2.4. Fatty Acid Analysis
2.5. Preparation of Fatty Acid Methyl Esters
2.6. Blood Collection and Hormone Quantification
2.7. Offspring (F1) Maintenance
2.8. Behavioral Assessment
2.8.1. Elevated Plus Maze (EPM)
2.8.2. Open Field (OF)
2.9. Statistical Analysis
3. Results
3.1. Maternal Food Intake during Lactation
3.2. Maternal Parameters at the End of Lactation
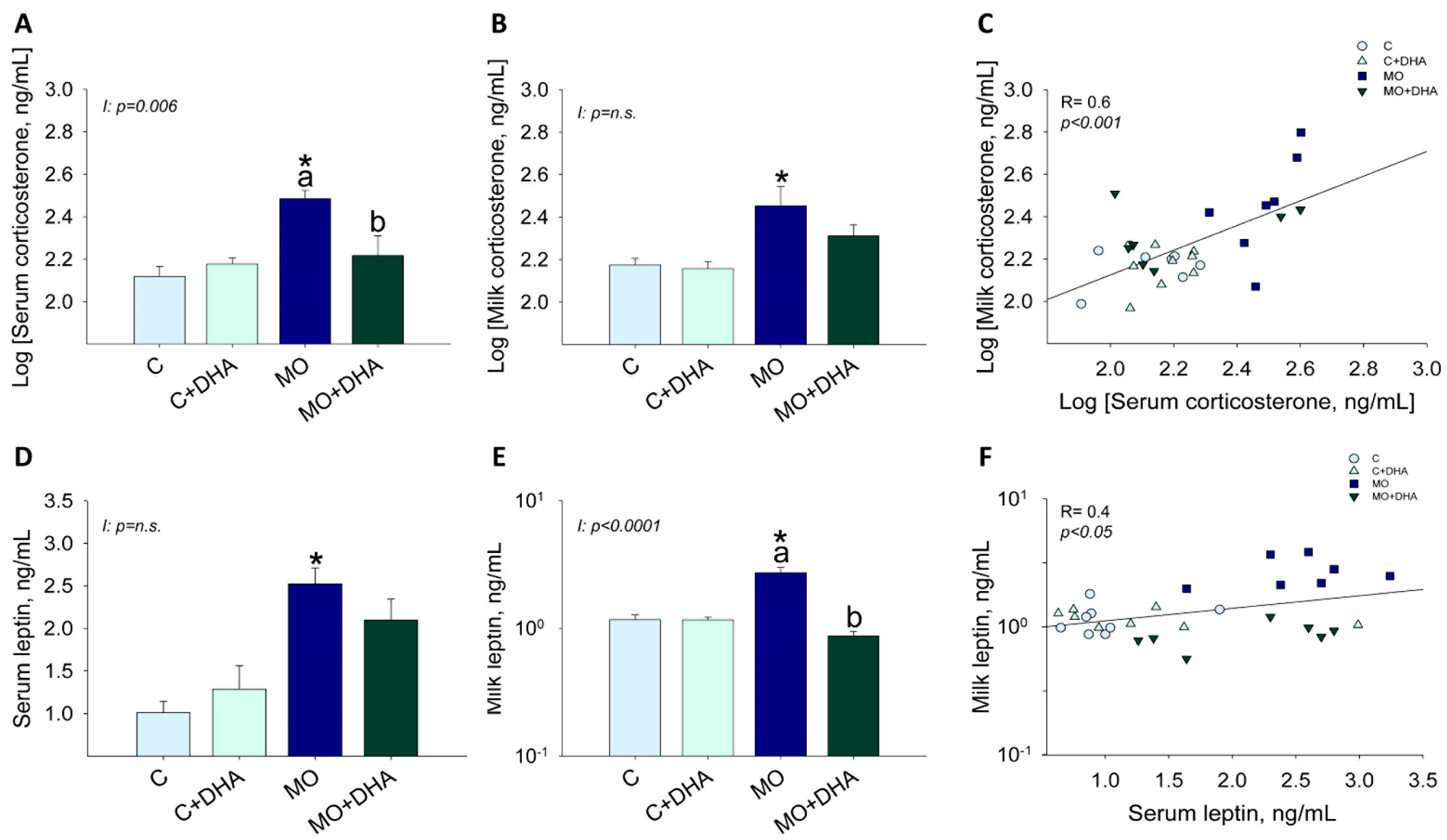
3.3. Maternal Hormonal Concentrations at the End of Lactation
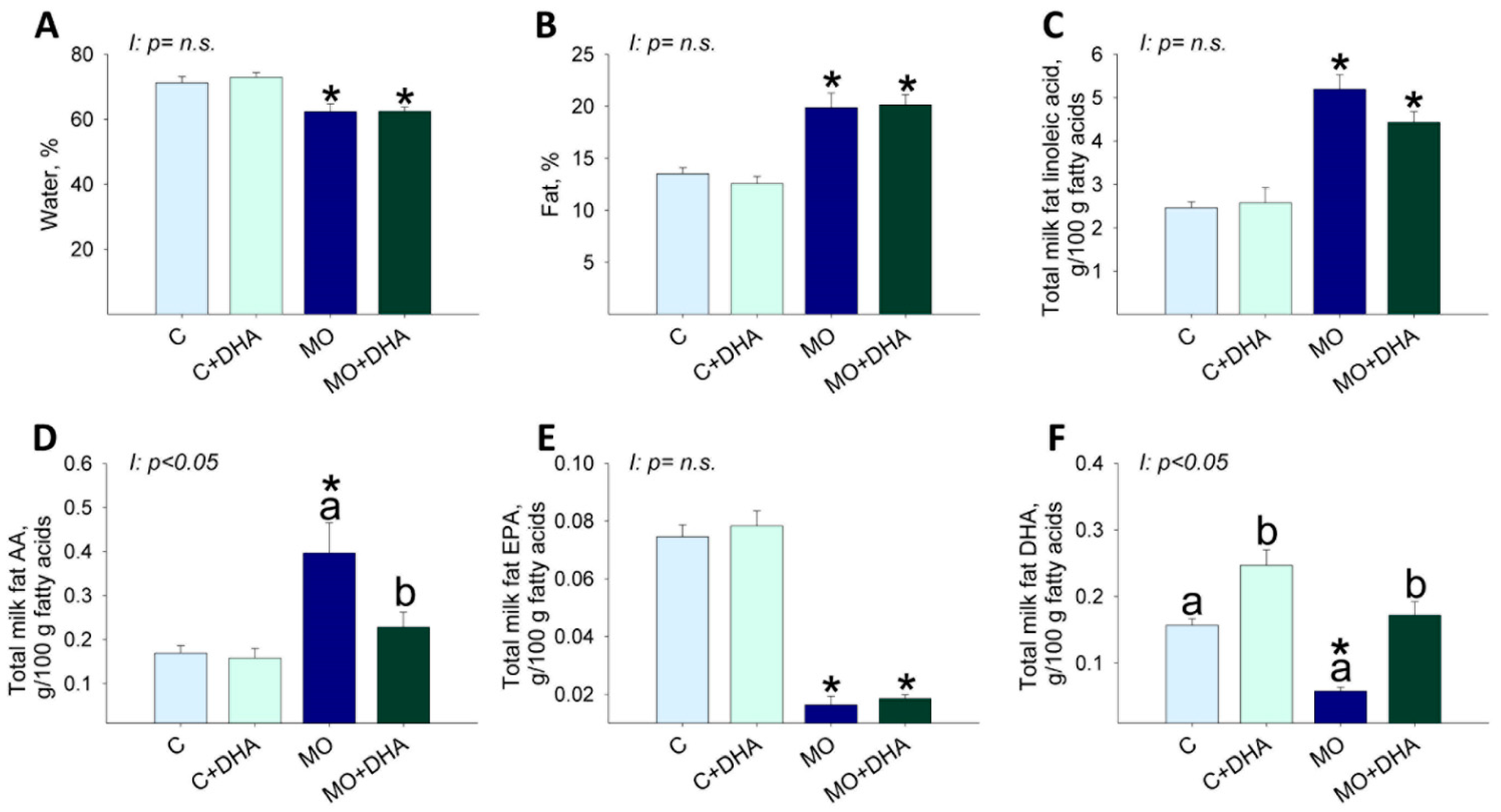
3.4. Milk Nutrient Composition at the End of Lactation
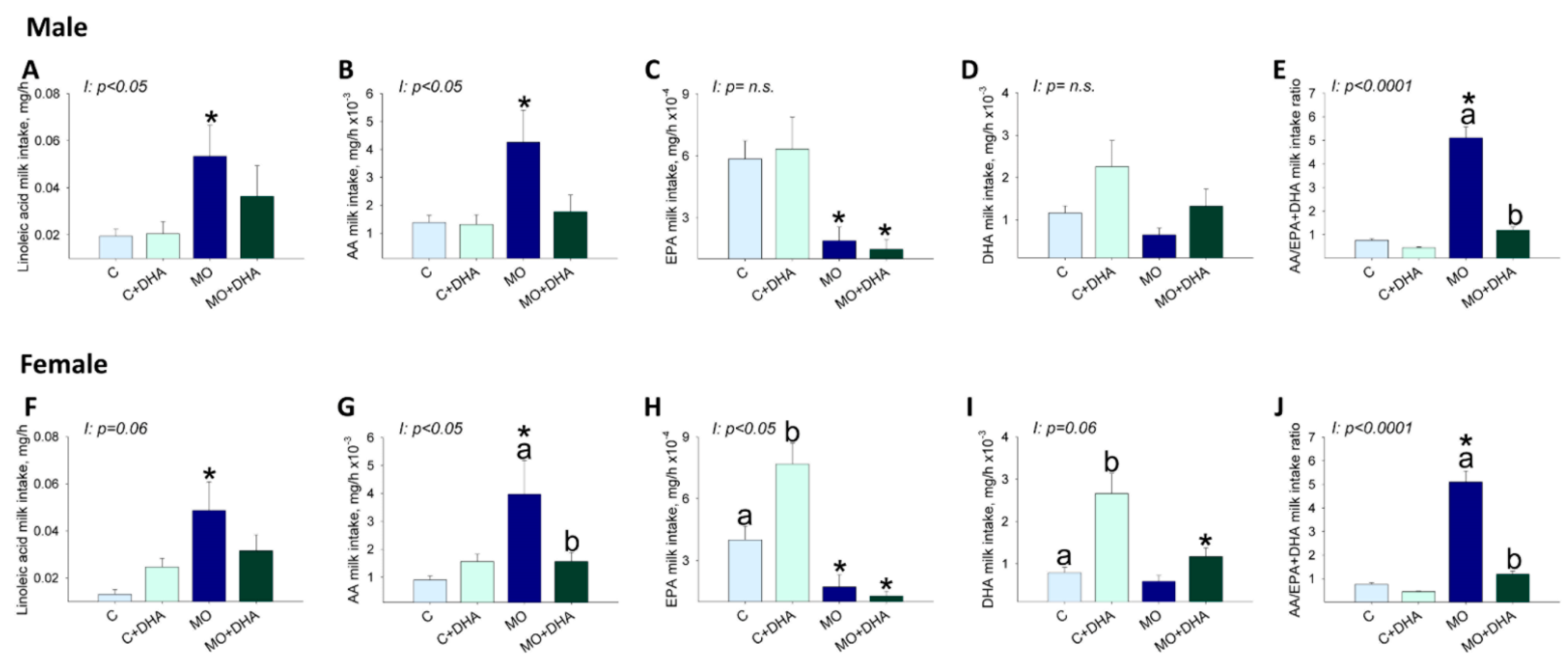
3.5. Pup Nutrient Intake in Milk at the End of Lactation
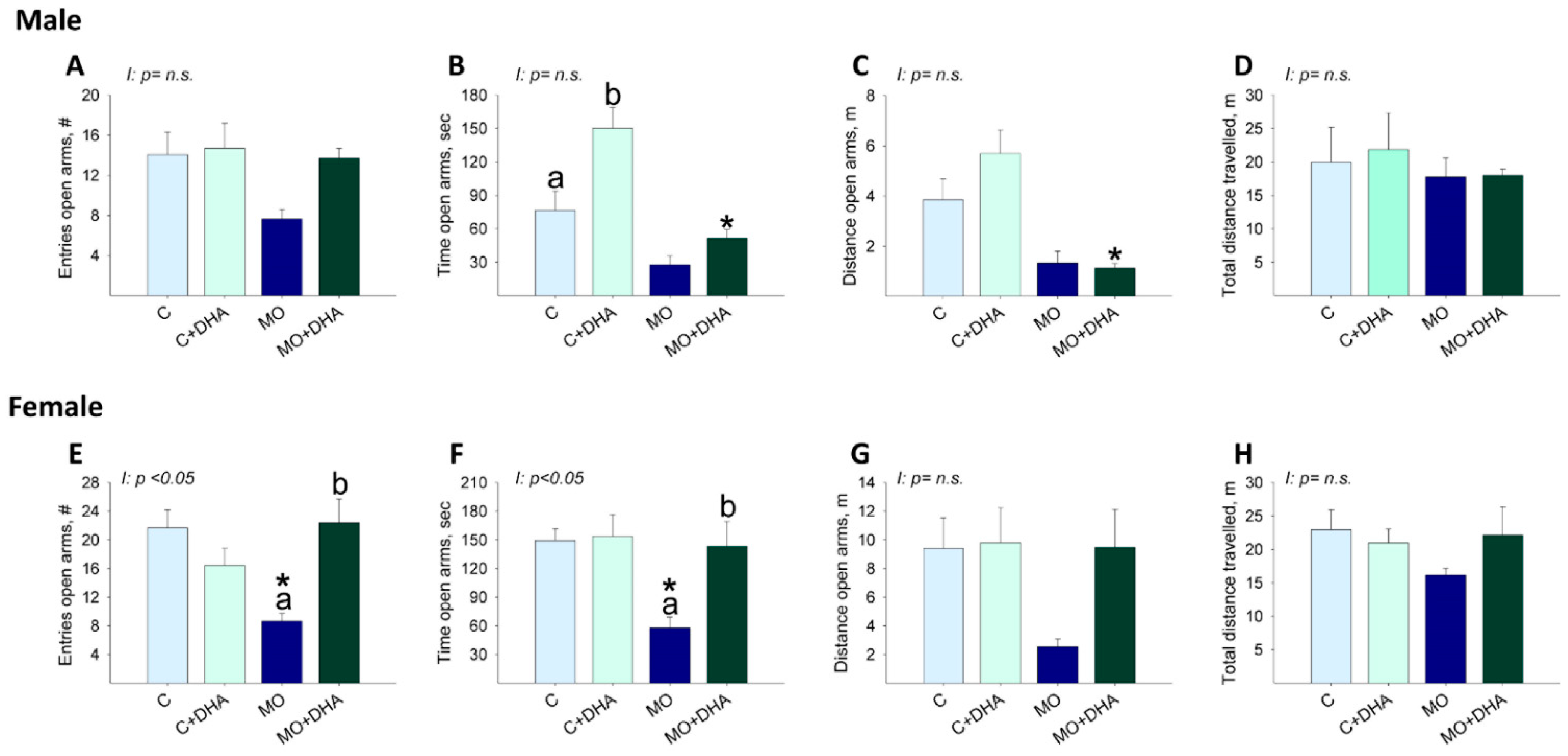
3.6. Elevated Plus Maze Studies
3.7. Open field Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Arredondo, A.; Torres, C.; Orozco, E.; Pacheco, S.; Aragon, A.; Huang, F.; Zambrano, E.; Bolanos-Jimenez, F. Socioeconomic determinants of maternal obesity in Mexico and France. Comparative analysis of two cohorts. Rev. Salud Publica 2018, 20, 245–253. [Google Scholar] [CrossRef]
- Arredondo, A.; Torres, C.; Orozco, E.; Pacheco, S.; Huang, F.; Zambrano, E.; Bolanos-Jimenez, F. Socio-economic indicators, dietary patterns, and physical activity as determinants of maternal obesity in middle-income countries: Evidences from a cohort study in Mexico. Int. J. Health Plan. Manag. 2019, 34, e713–e725. [Google Scholar] [CrossRef] [Green Version]
- Howie, G.J.; Sloboda, D.M.; Kamal, T.; Vickers, M.H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 2009, 587, 905–915. [Google Scholar] [CrossRef]
- Tsuduki, T.; Kitano, Y.; Honma, T.; Kijima, R.; Ikeda, I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J. Nutr. Sci. Vitaminol. 2013, 59, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Vieau, D. Perinatal nutritional programming of health and metabolic adult disease. World J. Diabetes 2011, 2, 133–136. [Google Scholar] [CrossRef]
- Andreasen, K.R.; Andersen, M.L.; Schantz, A.L. Obesity and pregnancy. Acta Obstet. Gynecol. Scand. 2004, 83, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Usha Kiran, T.S.; Hemmadi, S.; Bethel, J.; Evans, J. Outcome of pregnancy in a woman with an increased body mass index. BJOG 2005, 112, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Michaelsen, K.F.; Rasmussen, K.M.; Sorensen, T.I. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am. J. Clin. Nutr. 2004, 80, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Donath, S.M.; Amir, L.H. Does maternal obesity adversely affect breastfeeding initiation and duration? J. Paediatr. Child. Health 2000, 36, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Grammatikaki, E.; Kondaki, K.; Ioannou, E.; Anastasiadou, A.; Birbilis, M. The effect of maternal obesity on initiation and duration of breastfeeding in Greece: The GENESIS study. Public Health Nutr. 2009, 12, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, W.H. Breastfeeding may help prevent childhood overweight. JAMA 2001, 285, 2506–2507. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yue, Y.; Wang, H.; Zheng, J.; Chen, Z.; Chen, T.; Zhang, M.; Wang, S. Infant Breastfeeding and Behavioral Disorders in School-Age Children. Breastfeed. Med. 2019, 14, 115–120. [Google Scholar] [CrossRef]
- Rudolph, M.C.; Young, B.E.; Lemas, D.J.; Palmer, C.E.; Hernandez, T.L.; Barbour, L.A.; Friedman, J.E.; Krebs, N.F.; MacLean, P.S. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. 2017, 41, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [Green Version]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Salem, N., Jr.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.S.; Rodriguez-Gonzalez, G.L.; Reyes-Castro, L.A.; Ibanez, C.; Ramirez, A.; Chavira, R.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal obesity in the rat programs male offspring exploratory, learning and motivation behavior: Prevention by dietary intervention pre-gestation or in gestation. Int. J. Dev. Neurosci. 2012, 30, 75–81. [Google Scholar] [CrossRef]
- Zambrano, E.; Martinez-Samayoa, P.M.; Rodriguez-Gonzalez, G.L.; Nathanielsz, P.W. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J. Physiol. 2010, 588, 1791–1799. [Google Scholar] [CrossRef]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–1041S. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Winther, G.; Elfving, B.; Muller, H.K.; Lund, S.; Wegener, G. Maternal High-fat Diet Programs Offspring Emotional Behavior in Adulthood. Neuroscience 2018, 388, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Schmid, D.; Zang, K.; Much, D.; Knoeferl, B.; Kratzsch, J.; Amann-Gassner, U.; Bader, B.L.; Hauner, H. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr. Obes. 2015, 10, 67–73. [Google Scholar] [CrossRef]
- Grey, K.R.; Davis, E.P.; Sandman, C.A.; Glynn, L.M. Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology 2013, 38, 1178–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.J.; Neuringer, M.; Lin, D.S.; Connor, W.E. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr. Res. 2005, 58, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Armitage, J.A.; Pearce, A.D.; Sinclair, A.J.; Vingrys, A.J.; Weisinger, R.S.; Weisinger, H.S. Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids 2003, 38, 459–464. [Google Scholar] [CrossRef]
- Smithers, L.G.; Gibson, R.A.; McPhee, A.; Makrides, M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp. Physiol. 2015, 100, 755–758. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Gonzalez, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Beltran, A.A.; Ibanez, C.A.; Vega, C.C.; Lomas-Soria, C.; Castro-Rodriguez, D.C.; Elias-Lopez, A.L.; Nathanielsz, P.W.; et al. Maternal obesity accelerates rat offspring metabolic ageing in a sex-dependent manner. J. Physiol. 2019, 597, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, G.L.; Bautista, C.J.; Rojas-Torres, K.I.; Nathanielsz, P.W.; Zambrano, E. Importance of the lactation period in developmental programming in rodents. Nutr. Rev. 2020, 78, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.J.; Montano, S.; Ramirez, V.; Morales, A.; Nathanielsz, P.W.; Bobadilla, N.A.; Zambrano, E. Changes in milk composition in obese rats consuming a high-fat diet. Br. J. Nutr. 2016, 115, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helrich, K.; Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Arlington, VA, USA, 1990. [Google Scholar]
- Torres, N.; Bautista, C.J.; Tovar, A.R.; Ordaz, G.; Rodriguez-Cruz, M.; Ortiz, V.; Granados, O.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E270–E277. [Google Scholar] [CrossRef] [Green Version]
- Bautista, C.J.; Bautista, R.J.; Montaño, S.; Reyes-Castro, L.A.; Rodriguez-Peña, O.N.; Ibáñez, C.A.; Nathanielsz, P.W.; Zambrano, E. Effects of maternal protein restriction during pregnancy and lactation on milk composition and offspring development. Br. J. Nutr. 2019, 122, 141–151. [Google Scholar] [CrossRef]
- Romero, J.J.; Canas, R.; Baldwin, R.L. A technique for estimating milk production in rats. J. Nutr. 1975, 105, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.A. Milk yield, suckling behaviour and milk ejection in the lactating rat nursing litters of different sizes. J. Physiol. 1980, 303, 403–415. [Google Scholar] [CrossRef]
- Bautista, C.J.; Rodriguez-Gonzalez, G.L.; Torres, N.; Hernandez-Pando, R.; Ramirez, V.; Rodriguez-Cruz, M.; Nathanielsz, P.W.; Zambrano, E. Protein restriction in the rat negatively impacts long-chain polyunsaturated fatty acid composition and mammary gland development at the end of gestation. Arch. Med. Res. 2013, 44, 429–436. [Google Scholar] [CrossRef]
- Reyes-Castro, L.A.; Rodriguez, J.S.; Rodriguez-Gonzalez, G.L.; Chavira, R.; Bautista, C.J.; McDonald, T.J.; Nathanielsz, P.W.; Zambrano, E. Pre- and/or postnatal protein restriction developmentally programs affect and risk assessment behaviors in adult male rats. Behav. Brain Res. 2012, 227, 324–329. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C.A. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gonzalez, G.L.; Castro-Rodriguez, D.C.; Zambrano, E. Pregnancy and Lactation: A Window of Opportunity to Improve Individual Health. Methods Mol. Biol. 2018, 1735, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.C.; Reyes-Castro, L.A.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int. J. Obes. 2015, 39, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, A.M.; Garcia-Maldonado, E.; Gallego-Narbon, A.; Zapatera, B.; Vaquero, M.P. Fatty Acid Profile and Cardiometabolic Markers in Relation with Diet Type and Omega-3 Supplementation in Spanish Vegetarians. Nutrients 2019, 11, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapinski, A.; Sapinski, F. Determining blood pressure by the method of Doppler ultrasonography in the graphic version. Wiad. Lek. 1988, 41, 379–382. [Google Scholar] [PubMed]
- Cordner, Z.A.; Khambadkone, S.G.; Boersma, G.J.; Song, L.; Summers, T.N.; Moran, T.H.; Tamashiro, K.L.K. Maternal high-fat diet results in cognitive impairment and hippocampal gene expression changes in rat offspring. Exp. Neurol. 2019, 318, 92–100. [Google Scholar] [CrossRef]
- Roy, H.J.; Keenan, M.J.; Zablah-Pimentel, E.; Hegsted, M.; Bulot, L.; O’Neil, C.E.; Bunting, L.D.; Fernandez, J.M. Adult female rats defend “appropriate” energy intake after adaptation to dietary energy. Obes. Res. 2003, 11, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Bautista, C.J.; Guzman, C.; Rodriguez-Gonzalez, G.L.; Zambrano, E. Improvement in metabolic effects by dietary intervention is dependent on the precise nature of the developmental programming challenge. J. Dev. Orig. Health Dis. 2015, 6, 327–334. [Google Scholar] [CrossRef]
- Lien, E.L.; Boyle, F.G.; Wrenn, J.M.; Perry, R.W.; Thompson, C.A.; Borzelleca, J.F. Comparison of AIN-76A and AIN-93G diets: A 13-week study in rats. Food Chem. Toxicol. 2001, 39, 385–392. [Google Scholar] [CrossRef]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef]
- Zhuang, P.; Lu, Y.; Shou, Q.; Mao, L.; He, L.; Wang, J.; Chen, J.; Zhang, Y.; Jiao, J. Differential Anti-Adipogenic Effects of Eicosapentaenoic and Docosahexaenoic Acids in Obesity. Mol. Nutr. Food Res. 2019, 63, e1801135. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Schwartz, M.W. Clinical review: Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Del Rosso, L.; Mendoza, S.P.; Capitanio, J.P. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Van Doorn, C.; Macht, V.A.; Grillo, C.A.; Reagan, L.P. Leptin resistance and hippocampal behavioral deficits. Physiol. Behav. 2017, 176, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.M.; Plaut, K. The role of glucocorticoids in secretory activation and milk secretion, a historical perspective. J. Mammary Gland Biol. Neoplasia 2007, 12, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Bioactive factors in human milk. Pediatr. Clin. N. Am. 2001, 48, 69–86. [Google Scholar] [CrossRef]
- Hechler, C.; Beijers, R.; Riksen-Walraven, J.M.; de Weerth, C. Are cortisol concentrations in human breast milk associated with infant crying? Dev. Psychobiol. 2018, 60, 639–650. [Google Scholar] [CrossRef]
- Glynn, L.M.; Davis, E.P.; Schetter, C.D.; Chicz-Demet, A.; Hobel, C.J.; Sandman, C.A. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Hum. Dev. 2007, 83, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinel, A.L.; Rey, C.; Bonhomme, C.; Le Ruyet, P.; Joffre, C.; Laye, S. Dairy fat blend improves brain DHA and neuroplasticity and regulates corticosterone in mice. Prostaglandins Leukot. Essent. Fatty Acids 2016, 109, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, X.; Mick, G.J.; Kabarowski, J.H.; Wilson, L.S.; Barnes, S.; Walcott, G.P.; Luo, X.; McCormick, K. Effect of n-3 and n-6 Polyunsaturated Fatty Acids on Microsomal P450 Steroidogenic Enzyme Activities and In Vitro Cortisol Production in Adrenal Tissue From Yorkshire Boars. Endocrinology 2016, 157, 1512–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrieu, T.; Hilal, M.L.; Fourrier, C.; De Smedt-Peyrusse, V.; Sans, N.; Capuron, L.; Laye, S. Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozhenko, Y.; Asnani-Kishnani, M.; Rodriguez, A.M.; Palou, A. Milk Leptin Surge and Biological Rhythms of Leptin and Other Regulatory Proteins in Breastmilk. PLoS ONE 2015, 10, e0145376. [Google Scholar] [CrossRef] [Green Version]
- Savino, F.; Sardo, A.; Rossi, L.; Benetti, S.; Savino, A.; Silvestro, L. Mother and Infant Body Mass Index, Breast Milk Leptin and Their Serum Leptin Values. Nutrients 2016, 8, 383. [Google Scholar] [CrossRef]
- Khadge, S.; Thiele, G.M.; Sharp, J.G.; McGuire, T.R.; Klassen, L.W.; Black, P.N.; DiRusso, C.C.; Talmadge, J.E. Long-Chain Omega-3 Polyunsaturated Fatty Acids Modulate Mammary Gland Composition and Inflammation. J. Mammary Gland Biol. Neoplasia 2018, 23, 43–58. [Google Scholar] [CrossRef]
- Hollanders, J.J.; Heijboer, A.C.; van der Voorn, B.; Rotteveel, J.; Finken, M.J.J. Nutritional programming by glucocorticoids in breast milk: Targets, mechanisms and possible implications. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 397–408. [Google Scholar] [CrossRef]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef]
- Fraga, M.C.; de Moura, E.G.; da Silva Lima, N.; Lisboa, P.C.; de Oliveira, E.; Silva, J.O.; Claudio-Neto, S.; Filgueiras, C.C.; Abreu-Villaca, Y.; Manhaes, A.C. Anxiety-like, novelty-seeking and memory/learning behavioral traits in male Wistar rats submitted to early weaning. Physiol. Behav. 2014, 124, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.Y.; Barbieiri, P.; Castro, G.S.; Jordao, A.A., Jr.; Perdona Gda, S.; Sartorelli, D.S. Dietary polyunsaturated fatty acid intake during late pregnancy affects fatty acid composition of mature breast milk. Nutrition 2014, 30, 685–689. [Google Scholar] [CrossRef]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fatty Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.; Martin, J.C.; Agnani, G.; Pages, N.; Leruyet, P.; Carayon, P.; Delplanque, B. Dairy fat blends high in alpha-linolenic acid are superior to n-3 fatty-acid-enriched palm oil blends for increasing DHA levels in the brains of young rats. J. Nutr. Biochem. 2012, 23, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Du, Q.; Agnani, G.; Le Ruyet, P.; Martin, J.C. A dairy fat matrix providing alpha-linolenic acid (ALA) is better than a vegetable fat mixture to increase brain DHA accretion in young rats. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, Polyunsaturated Fatty Acid Levels in Colostrum and Child Intelligence Quotient at Age 5–6 Years. J. Pediatr. 2017, 183, 43–50.e43. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Bohles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr. Res. 2007, 61, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, D.; Tran, N.; Peach, J.; Edwards, T.; Greeley, M. Toxicologic evaluations of DHA-rich algal oil in rats: Developmental toxicity study and 3-month dietary toxicity study with an in utero exposure phase. Food Chem. Toxicol. 2012, 50, 4149–4157. [Google Scholar] [CrossRef]
- Whelan, J.; Whelan, J. Conversion of dietary polyunsaturated fats between humans and rodents: A review of allometric scaling models. Prostaglandins Leukot. Essent. Fatty Acids 2020, 158, 102094. [Google Scholar] [CrossRef]
- Gao, J.; Wu, H.; Cao, Y.; Liang, S.; Sun, C.; Wang, P.; Wang, J.; Sun, H.; Wu, L. Maternal DHA supplementation protects rat offspring against impairment of learning and memory following prenatal exposure to valproic acid. J. Nutr. Biochem. 2016, 35, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.; Drover, J.R.; Castaneda, Y.S.; Minns, L.; Wheaton, D.K.; Mundy, D.; et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colson, C.; Ghandour, R.A.; Dufies, O.; Rekima, S.; Loubat, A.; Munro, P.; Boyer, L.; Pisani, D.F. Diet Supplementation in omega3 Polyunsaturated Fatty Acid Favors an Anti-Inflammatory Basal Environment in Mouse Adipose Tissue. Nutrients 2019, 11, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Lundqvist-Persson, C.; Lau, G.; Nordin, P.; Strandvik, B.; Sabel, K.G. Early behaviour and development in breastfed premature infants are influenced by omega-6 and omega-3 fatty acid status. Early Hum. Dev. 2010, 86, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, M.; Gabrielsson, B.G.; Holmang, A.; Larsson, B.M.; Hanson, L.A.; Strandvik, B. Gender-related long-term effects in adult rats by perinatal dietary ratio of n-6/n-3 fatty acids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R575–R579. [Google Scholar] [CrossRef]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Aspects Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- Das, U.N. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease--but how and why? Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 11–19. [Google Scholar] [CrossRef]
- Gomez Candela, C.; Bermejo Lopez, L.M.; Loria Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef]
- Shors, T.J.; Chua, C.; Falduto, J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001, 21, 6292–6297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armario, A.; Castellanos, J.M. Effect of acute and chronic stress on testosterone secretion in male rats. J. Endocrinol. Investig. 1984, 7, 659–661. [Google Scholar] [CrossRef]
- Legrand, C.; Vivat, V.; Rigolot, C.; Maltier, J.P. Selective distribution of alpha-1 and beta adrenoceptors in pregnant rat uterus visualized by autoradiography. J. Pharmacol. Exp. Ther. 1991, 256, 767–772. [Google Scholar] [PubMed]
- Abbott, K.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. Dietary supplementation with docosahexaenoic acid rich fish oil increases circulating levels of testosterone in overweight and obese men. Prostaglandins Leukot. Essent. Fatty Acids 2020, 163, 102204. [Google Scholar] [CrossRef] [PubMed]
- Demers, G.; Roy, J.; Machuca-Parra, A.I.; Dashtehei Pour, Z.; Bairamian, D.; Daneault, C.; Rosiers, C.D.; Ferreira, G.; Alquier, T.; Fulton, S.; et al. Fish oil supplementation alleviates metabolic and anxiodepressive effects of diet-induced obesity and associated changes in brain lipid composition in mice. Int. J. Obes. 2020, 44, 1936–1945. [Google Scholar] [CrossRef]
- Lam, V.Y.Y.; Raineki, C.; Wang, L.Y.; Chiu, M.; Lee, G.; Ellis, L.; Yu, W.; Weinberg, J. Role of corticosterone in anxiety- and depressive-like behavior and HPA regulation following prenatal alcohol exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 1–15. [Google Scholar] [CrossRef]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gopaldas, M.; Zanderigo, F.; Zhan, S.; Ogden, R.T.; Miller, J.M.; Rubin-Falcone, H.; Cooper, T.B.; Oquendo, M.A.; Sullivan, G.; Mann, J.J.; et al. Brain serotonin transporter binding, plasma arachidonic acid and depression severity: A positron emission tomography study of major depression. J. Affect. Disord. 2019, 257, 495–503. [Google Scholar] [CrossRef] [PubMed]



| Fatty Acids, % | C | C+DHA | MO | MO+DHA | Interaction |
|---|---|---|---|---|---|
| ω-3 PUFAS | 0.4 ± 0.01 | 0.5 ± 0.08 | 0.4 ± 0.03 | 0.4 ± 0.03 | p = n.s. |
| ω-6 PUFAS | 2.8 ± 0.16 | 2.9 ± 0.39 | 5.7 ± 0.51 * | 4.9 ± 0.29 * | p = n.s. |
| MSA | 4.6 ± 0.29 | 3.5 ± 0.52 | 6.1 ± 1.42 | 7.7 ± 0.38 * | p = 0.06 |
| SFA | 5.8 ± 0.18 | 5.7 ± 0.44 | 7.6 ± 0.54 * | 7.1 ± 0.34 | p = n.s. |
| Fatty acids, mg/h | C | C+DHA | MO | MO+DHA | Interaction |
|---|---|---|---|---|---|
| Male | |||||
| ω-3 PUFAS | 3 ± 0.4 | 4 ± 1 | 4 ± 0.9 | 4 ± 1 | p = n.s. |
| ω-6 PUFAS | 20 ± 3 | 20 ± 6 | 60 ± 10 * | 40 ± 10 | p = n.s. |
| ω-6/ ω-3 ratio | 7.4 ± 0.36 a | 5.7 ± 0.17 b | 15.8 ± 0.42 a* | 11.2 ± 0.54 b* | p < 0.05 |
| MSA | 36 ± 6 | 29 ± 9 | 68 ± 29 | 64 ± 23 | p = n.s. |
| SFA | 40 ± 6 | 50 ± 10 | 80 ± 20 | 60 ± 20 | p = n.s. |
| Female | |||||
| ω-3 PUFAS | 2 ± 0.3 a | 5 ± 0.8 b | 3 ± 0.7 | 3 ± 0.7 | p < 0.05 |
| ω-6 PUFAS | 10 ± 2 | 30 ± 4 | 60 ± 10 * | 40 ± 7 | p < 0.05 |
| ω-6/ω-3 ratio | 7.4 ± 0.36 a | 5.7 ± 0.17 b | 15.8 ± 0.42 a* | 11.2 ± 0.54 b* | p < 0.05 |
| MSA | 24 ± 4 | 36 ± 8 | 58 ± 23 | 56 ± 12 | p = n.s. |
| SFA | 30 ± 4 | 50 ± 6 | 70 ± 20* | 50 ± 10 | p < 0.05 |
| C | C+DHA | MO | MO+DHA | Interaction | |
|---|---|---|---|---|---|
| Male | |||||
| Total distance, m | 47.1 ± 1.6 | 46.1 ± 3.5 | 44.9 ± 6.9 | 56.1 ± 9.8 | p = n.s. |
| Center zone entries, # | 10.2 ± 1.7 | 8.8 ± 1.1 | 3.0 ± 0.4 * | 7.7± 1.9 | p = 0.06 |
| Center zone time, s | 13.2 ± 1.6 | 13.0 ± 2.3 | 4.3 ± 1.5 | 12.9 ± 5.3 | p = n.s. |
| Center zone distance, m | 2.9 ± 0.6 | 2.2 ± 0.3 | 0.87 ± 0.3 * | 2.0 ± 0.4 | p = 0.06 |
| Female | |||||
| Total distance, m | 57.8 ± 3.8 | 62.3 ± 2.7 | 49.2 ± 3.5 | 61.5 ± 4.9 | p = n.s. |
| Center zone entries, # | 13.0 ± 2.9 | 13.7 ± 2.1 | 7.7 ± 1.9 | 12.6 ± 2.5 | p = n.s. |
| Center zone time, s | 21.1 ± 5.0 | 22.6 ± 5.1 | 15.9 ± 4.7 | 20.7 ± 4.5 | p = n.s. |
| Center zone distance, m | 4.1 ± 0.9 | 3.7 ± 0.7 | 2.3 ± 0.6 | 3.8 ± 0.5 | p = n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, E.; Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Castro-Rodríguez, D.C.; Juárez-Pilares, G.; Ibáñez, C.A.; Hernández-Rojas, A.; Nathanielsz, P.W.; Montaño, S.; et al. DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring. Nutrients 2021, 13, 4243. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124243
Zambrano E, Rodríguez-González GL, Reyes-Castro LA, Bautista CJ, Castro-Rodríguez DC, Juárez-Pilares G, Ibáñez CA, Hernández-Rojas A, Nathanielsz PW, Montaño S, et al. DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring. Nutrients. 2021; 13(12):4243. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124243
Chicago/Turabian StyleZambrano, Elena, Guadalupe L. Rodríguez-González, Luis A. Reyes-Castro, Claudia J. Bautista, Diana C. Castro-Rodríguez, Gimena Juárez-Pilares, Carlos A. Ibáñez, Alejandra Hernández-Rojas, Peter W. Nathanielsz, Sara Montaño, and et al. 2021. "DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring" Nutrients 13, no. 12: 4243. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124243







