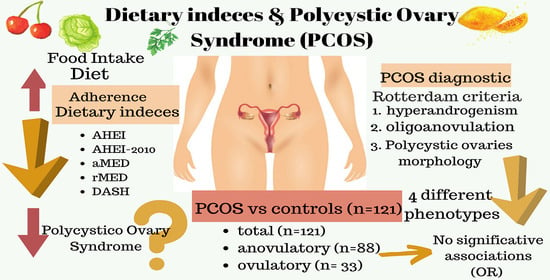
Are Dietary Indices Associated with Polycystic Ovary Syndrome and Its Phenotypes? A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
- taking medication that interferes carbohydrate metabolism, not excluded previously (cases = 100; controls = 134)
- taking multivitamins 10–12 months (cases = 98; controls = 131)
- taking multivitamins 4–6 months or 10–12 months (cases = 90; controls = 123)
- oligovulation/amenorrhea or anovulation (menstrual cycles > 35 days or amenorrhea > 3 months).
- biochemical hyperandrogenism (total testosterone level ≥ 2.6 nmol or clinical (Ferriman-Galwey score ≥ 12) [40].
- polycystic ovaries morphology (POM) using transvaginal ultrasound (TVUS) (≥12 follicles measuring 2–9 mm in diameter, mean of both ovaries) [41].
- hyperandrogenism + oligo/amenorrhea + POM (H + O + POM) (n = 52).
- hyperandrogenism + oligo/amenorrhea (H + O) (n = 18).
- hyperandrogenism + POM (H + POM) (n = 33).
- oligo/amenorrhea + POM (O + POM) (n = 18).
2.1. Dietary Assessment and Dietary Indices
- AHEI
- AHEI-2010
- rMED
- aMED
- DASH.
2.2. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCOS | Polycystic Ovarian Syndrome |
| NIH | National Institute of Health |
| European | Society of Human Reproduction and Embryology ASRM American |
| aMED | Alternate Mediterranean Diet Score |
| BMI | Body Mass Index |
| rMED | Relative Mediterranean Diet Score |
| AHEI | Alternate Healthy Index |
| AHEI-2010 | Alternate Healthy Index 2010 |
| H | Hyperandrogenism |
| DASH | Dietary Approaches to Stop Hypertension |
| O | Oligo/amenorrhea |
| POM | Polycystic ovaries morphology |
| H + O | “Hyperandorgenism + Oligo/amenorrhea” phenotype |
| H + O + POM | “Hyperandorgenism + Oligo/amenorrhea + Polycystic ovaries morphology” phenotype |
| H + O | “Hyperandorgenism + Oligo/amenorrhea” phenotype |
| O + POM | “Oligo/amenorrhea + Polycystic ovaries morphology” phenotype |
References
- Rothenberg, S.S.; Beverley, R.; Barnard, E.; Baradaran-Shoraka, M.; Sanfilippo, J.S. Polycystic ovary syndrome in adolescents. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Tziomalos, K.; Papadakis, E.; Vosnakis, C.; Chatzis, P.; Katsikis, I. Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: Impact on metabolism and fertility. Endocrine 2013, 44, 583–590. [Google Scholar] [CrossRef]
- Liu, A.L.; Xie, H.J.; Xie, H.Y.; Liu, J.; Yin, J.; Hu, J.S.; Peng, C.Y. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. Genet. 2017, 18, 89. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Nese, C.; Palomba, S.; Savastano, S.; Tafuri, D.; Colarieti, G.; La Sala, G.; Colao, A.; Yildiz, B.O. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: An uptodate in the management of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 214–219. [Google Scholar] [CrossRef]
- Mani, H.; Levy, M.J.; Davies, M.J.; Morris, D.H.; Gray, L.J.; Bankart, J.; Blackledge, H.; Khunti, K.; Howlett, T.A. Diabetes and cardiovascular events in women with polycystic ovary syndrome: A 20-year retrospective cohort study. Clin. Endocrinol. Oxf. 2013, 78, 926–934. [Google Scholar] [CrossRef]
- Albu, A.; Radian, S.; Fica, S.; Barbu, C.G. Biochemical hyperandrogenism is associated with metabolic syndrome independently of adiposity and insulin resistance in Romanian polycystic ovary syndrome patients. Endocrine 2015, 48, 696–704. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Schwetz, V.; Giuliani, A.; Obermayer-Pietsch, B. Assessment of glucose metabolism in polycystic ovary syndrome: HbA1c or fasting glucose compared with the oral glucose tolerance test as a screening method. Hum. Reprod. 2013, 28, 2537–2544. [Google Scholar] [CrossRef]
- Daan, N.M.P.; Louwers, Y.V.; Koster, M.P.H.; Eijkemans, M.J.C.; de Rijke, Y.B.; Lentjes, E.W.G.; Fauser, B.C.J.M.; Laven, J.S.E. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: Who is really at risk? Fertil. Steril. 2014, 102, 1444–1451.e3. [Google Scholar] [CrossRef]
- Lo, J.C.; Feigenbaum, S.L.; Yang, J.; Pressman, A.R.; Selby, J.V.; Go, A.S. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1357–1363. [Google Scholar] [CrossRef]
- Chiu, W.-L.; Boyle, J.; Vincent, A.; Teede, H.; Moran, L.J. Cardiometabolic Risks in Polycystic Ovary Syndrome: Non-Traditional Risk Factors and the Impact of Obesity. Neuroendocrinology 2017, 104, 412–424. [Google Scholar] [CrossRef]
- Morgante, G.; Musacchio, M.C.; Orvieto, R.; Massaro, M.G.; De Leo, V. Alterations in thyroid function among the different polycystic ovary syndrome phenotypes. Gynecol. Endocrinol. 2013, 29, 967–969. [Google Scholar] [CrossRef]
- Garelli, S.; Masiero, S.; Plebani, M.; Chen, S.; Furmaniak, J.; Armanini, D.; Betterle, C. High prevalence of chronic thyroiditis in patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 248–251. [Google Scholar] [CrossRef]
- Joham, A.E.; Teede, H.J.; Ranasinha, S.; Zoungas, S.; Boyle, J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: Data from a large community-based cohort study. J. Women’s. Health Larchmt 2015, 24, 299–307. [Google Scholar] [CrossRef]
- West, S.; Lashen, H.; Bloigu, A.; Franks, S.; Puukka, K.; Ruokonen, A.; Jarvelin, M.-R.; Tapanainen, J.S.; Morin-Papunen, L. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum. Reprod. 2014, 29, 2339–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helseth, R.; Vanky, E.; Salvesen, O.; Carlsen, S.M. Gestational diabetes mellitus among Norwegian women with polycystic ovary syndrome: Prevalence and risk factors according to the WHO and the modified IADPSG criteria. Eur. J. Endocrinol. 2013, 169, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Joham, A.E.; Ranasinha, S.; Zoungas, S.; Moran, L.; Teede, H.J. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E447–E452. [Google Scholar] [CrossRef]
- Fux-Otta, C.; Maliqueo, M.; Echiburú, B.; Rosato, O.; Crisosto, N.; Iraci, G.S.; Fiol de Cuneo, M.; Szafryk de Mereshian, P.; Sir-Petermann, T. Pregnancy outcomes in women with polycystic ovary syndrome in two Latin American populations. J. Obstet. Gynaecol. 2018, 38, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Atabiekov, I.; Azziz, R. Polycystic Ovary Syndrome. In Reference Module in Biomedical Sciences; Elsevier: Oxford, UK, 2018; ISBN 9780128012383. [Google Scholar]
- Lansdown, A.; Rees, D.A. The sympathetic nervous system in polycystic ovary syndrome: A novel therapeutic target? Clin. Endocrinol. (Oxf.) 2012, 77, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, L.G.; Lee, I.; Sammel, M.D.; Dokras, A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017, 32, 1075–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslamian, G.; Baghestani, A.-R.; Eghtesad, S.; Hekmatdoost, A. Dietary carbohydrate composition is associated with polycystic ovary syndrome: A case-control study. J. Hum. Nutr. Diet. 2017, 30, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2019, 2019, 4386401. [Google Scholar] [CrossRef] [PubMed]
- McGrice, M.; Porter, J. The effect of low carbohydrate diets on fertility hormones and outcomes in overweight and obese women: A systematic review. Nutrients 2017, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Phy, J.L.; Pohlmeier, A.M.; Cooper, J.A.; Watkins, P.; Spallholz, J.; Harris, K.S.; Berenson, A.B.; Boylan, M. Low Starch/Low Dairy Diet Results in Successful Treatment of Obesity and Co-Morbidities Linked to Polycystic Ovary Syndrome (PCOS). J. Obes. Weight Loss Ther. 2015, 5, 259. [Google Scholar] [CrossRef] [Green Version]
- Pohlmeier, A.M.; Phy, J.L.; Watkins, P.; Boylan, M.; Spallholz, J.; Harris, K.S.; Cooper, J.A. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Appl. Physiol. Nutr. Metab. 2014, 39, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef]
- Perelman, D.; Coghlan, N.; Lamendola, C.; Carter, S.; Abbasi, F.; McLaughlin, T. Substituting poly- and mono-unsaturated fat for dietary carbohydrate reduces hyperinsulinemia in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2017, 33, 324–327. [Google Scholar] [CrossRef]
- Azadi-Yazdi, M.; Karimi-Zarchi, M.; Salehi-Abargouei, A.; Fallahzadeh, H.; Nadjarzadeh, A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 275–283. [Google Scholar] [CrossRef]
- Foroozanfard, F.; Rafiei, H.; Samimi, M.; Gilasi, H.R.; Gorjizadeh, R.; Heidar, Z.; Asemi, Z. The effects of dietary approaches to stop hypertension diet on weight loss, anti-Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: A randomized clinical trial. Clin. Endocrinol. Oxf. 2017, 87, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Esmaillzadeh, A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm. Metab. Res. 2015, 47, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Sabihi, S.-S.; Esmaillzadeh, A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized clinical trial. Nutrition 2014, 30, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, M.L.; Mendiola, J.; Hernández-Peñalver, A.I.; Corbalán-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sánchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum. Reprod. 2017, 32, 2315–2323. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Peñalver, A.I.; Sánchez-Ferrer, M.L.; Mendiola, J.; Adoamnei, E.; Prieto-Sánchez, M.T.; Corbalán-Biyang, S.; Carmona-Barnosi, A.; Nieto, A.; Torres-Cantero, A.M. Assessment of anogenital distance as a diagnostic tool in polycystic ovary syndrome. Reprod. Biomed. Online 2018, 37, 741–749. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Prieto-Sánchez, M.T.; Corbalán-Biyang, S.; Mendiola, J.; Adoamnei, E.; Hernández-Peñalver, A.I.; Carmona-Barnosi, A.; Salido-Fiérrez, E.J.; Torres-Cantero, A.M. Are there differences in basal thrombophilias and C-reactive protein between women with or without PCOS? Reprod. Biomed. Online 2019, 38, 1018–1026. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Adoamnei, E.; Prieto-Sánchez, M.T.; Mendiola, J.; Corbalán-Biyang, S.; Moñino-García, M.; Palomar-Rodríguez, J.A.; Torres-Cantero, A.M. Health-related quality of life in women with polycystic ovary syndrome attending to a tertiary hospital in Southeastern Spain: A case-control study. Health Qual. Life Outcomes 2020, 18, 232. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Afifi, L.; Saeed, L.; Pasch, L.A.; Huddleston, H.G.; Cedars, M.I.; Zane, L.T.; Shinkai, K. Association of ethnicity, Fitzpatrick skin type, and hirsutism: A retrospective cross-sectional study of women with polycystic ovarian syndrome. Int. J. Women’s Dermatol. 2017, 3, 37–43. [Google Scholar] [CrossRef]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome; National Institutes of Health: Bethesda, MD, USA, 2012; pp. 1–14.
- García-Arenzana, N.; Navarrete-Muñoz, E.M.; Vázquez-Carrete, J.A.; Moreno, P.; Vidal, C.; Salas, D.; Ederra, M.; Pedraz, C.; Collado-García, F.; Sánchez-Contador, C.; et al. Compliance with current dietary recommendations and geographical variability of diet in women participa-ting in 7 screening programs for breast cancer in spain. Nutr. Hosp. 2011, 26, 863–873. [Google Scholar] [CrossRef] [PubMed]
- García-Arenzana, N.; Navarrete-Muñoz, E.M.; Peris, M.; Salas, D.; Ascunce, N.; Gonzalez, I.; Sánchez-Contador, C.; Santamariña, C.; Moreo, P.; Moreno, M.P.; et al. Diet quality and related factors among Spanish female participants in breast cancer screening programs. Menopause J. N. Am. Menopause Soc. 2012, 19, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Palma, I.; Farran, A.; Cantós, D. Tablas de Composición de Alimentos del CESNID; McGraw-Hill/Interamericana de España: Madrid, Spain, 2008; ISBN 9788448160906. [Google Scholar]
- US Department of Health and Human Services. 2015–2020 Dietary Guidelines. Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/#subnav-4 (accessed on 23 November 2020).
- Cutillas-Tolín, A.; Adoamnei, E.; Navarrete-Muñoz, E.M.; Vioque, J.; Moñino-García, M.; Jørgensen, N.; Chavarro, J.E.; Mendiola, J.; Torres-Cantero, A.M. Adherence to diet quality indices in relation to semen quality and reproductive hormones in young men. Hum. Reprod. 2019, 34, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.A.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- US Department of Agriculture. Dietary Guidelines for Americans; US Department of Health and Human Services, US Department of Agriculture: Washington, DC, USA, 1995.
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Obarzanek, E.; Windhauser, M.M.; Svetkey, L.P.; Vollmer, W.M.; McCullough, M.; Karanja, N.; Lin, P.H.; Steele, P.; Proschan, M.A.; et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann. Epidemiol. 1995, 5, 108–118. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the mediterranean diet and risk of coronary heart disease in the spanish EPIC cohort study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 14th ed.; Oxford University Press: Oxford, UK, 2013; ISBN 9780199979448. [Google Scholar]
- Lin, A.W.; Kazemi, M.; Jarrett, B.Y.; Vanden Brink, H.; Hoeger, K.M.; Spandorfer, S.D.; Lujan, M.E. Dietary and physical activity behaviors in women with polycystic ovary syndrome per the new international evidence-based guideline. Nutrients 2019, 11, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos S. Rodrigues, A.M.; Martins, L.B.; Franklin, A.M.T.; Candido, A.L.; dos Santos, L.C.; Ferreira, A.V.M. Poor quality diet is associated with overweight status and obesity in patients with polycystic ovary syndrome. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 2), 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, S.; Li, R.; Liu, P.; Qiao, J.; Zhang, Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: A meta-analysis. Reprod. Biol. Endocrinol. 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd. 2020, 80, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Andersen, M.; Azziz, R.; et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, G.W.; Legro, R.S. Longterm management of Polycystic Ovarian Syndrome (PCOS). Mol. Cell. Endocrinol. 2013, 373, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 2019, 1–90. [Google Scholar] [CrossRef]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.; Buckley, J.; Brinkworth, G.; Thomson, R.; Norman, R.J. Predictors of lifestyle intervention attrition or weight loss success in women with polycystic ovary syndrome who are overweight or obese. Nutrients 2019, 11, 492. [Google Scholar] [CrossRef] [Green Version]
- Garad, R.M.; Teede, H.J. Polycystic ovary syndrome: Improving policies, awareness, and clinical care. Curr. Opin. Endocr. Metab. Res. 2020, 12, 112–118. [Google Scholar] [CrossRef]
- Lie Fong, S.; Douma, A.; Verhaeghe, J. Implementing the international evidence-based guideline of assessment and management of polycystic ovary syndrome (PCOS): How to achieve weight loss in overweight and obese women with PCOS? J. Gynecol. Obstet. Hum. Reprod. 2020, in press. [Google Scholar] [CrossRef]
- Amirjani, S.; Asemi, Z.; Bazarganipour, F.; Aramesh, S.; Allan, H.; Sayadi, M.; Tabatabaei, M.-S.; Mohamadian, Z.; Zabti, F.; Iranpak, N.; et al. Dietary intake and lifestyle behaviour in different phenotypes of polycystic ovarian syndrome: A case–control study. J. Hum. Nutr. Diet. 2019, 32, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Brown, W.J.; McNaughton, S.A.; Joham, A.E.; Teede, H.J. Weight management practices associated with PCOS and their relationships with diet and physical activity. Hum. Reprod. 2017, 32, 669–978. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Patil, A.N.; Gudi, A.; Homburg, R.; Conway, G.S. Changes in diet composition with urbanization and its effect on the polycystic ovarian syndrome phenotype in a Western Indian population. Fertil. Steril. 2019, 112, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019, 236, 116940. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Chavarro, J.E.; Souter, I. Diet and female fertility: Doctor, what should I eat? Fertil. Steril. 2018, 110, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and Lifestyle in the Prevention of Ovulatory Disorder Infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; McBreairty, L.E.; Chizen, D.R.; Pierson, R.A.; Chilibeck, P.D.; Zello, G.A. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: A randomized controlled trial. Nutrients 2018, 10, 1387. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Ko, H.; Misso, M.; Marsh, K.; Noakes, M.; Talbot, M.; Frearson, M.; Thondan, M.; Stepto, N.; Teede, H.J. Dietary Composition in the Treatment of Polycystic Ovary Syndrome: A Systematic Review to Inform Evidence-Based Guidelines. J. Acad. Nutr. Diet. 2013, 113, 520–545. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [Green Version]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef] [Green Version]

| Category of Scoring Dietary Indices | AHEI 1 | AHEI-2010 | aMED | rMED | DASH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min 2 (0) | Max 3(10) | Min (0) | Max (10) | Min (0) | Max (1) | Min (0) | Max (2) | Min (0) | Max (5) | |
| Vegetables | 0 | 5 servings/day | 0 | ≥5 servings/day | <median servings/day | >median servings/day | Lowest tertile | Greatest tertile | Lowest quintile | Greatest quintile |
| Fruit | 0 | 4 servings/day | 0 | >4 servings/day | <median servings/day | >median servings/day | Lowest tertile | Greatest tertile | Lowest quintile | Greatest quintile |
| Nuts | 0 | 1 servings/day | <median servings/day | >median servings/day | Lowest tertile | Greatest tertile | ||||
| Nuts and legumes | 0 | ≥1 servings/day | Lowest quintile | Greatest quintile | ||||||
| Legumes | <median servings/day | >median servings/day | Lowest tertile | Greatest tertile | ||||||
| Cereals | ||||||||||
| Cereal fiber | 0 | 15 g/day | ||||||||
| Whole grains | 0 | 75 g/day | <median servings/day | >median servings/day | Lowest quintile | Greatest quintile | ||||
| Cereals | Lowest tertile | Greatest tertile | ||||||||
| Meat | ||||||||||
| Ratio of white to red meat | 0 | 4 | ||||||||
| Red and processed meat | ≥1.5 | 0 servings/day | >median servings/day | <median servings/day | ||||||
| Meat | Greatest tertile | Lowest tertile | ||||||||
| Fats | ||||||||||
| Trans fat | ≥4 | ≤0.5% of energy | ≥4 | ≤0.5% of energy | ||||||
| Ratio of saturated to polyunsaturated | ≥4 | ≥1 | ||||||||
| Ratio of monosaturated to saturated | <median servings/day | >median servings/day | ||||||||
| Polyunsaturated | ≤2 | ≥10% of energy | ||||||||
| ω 3 fats (EPA + DHA) 4 | 0 | 250 mg/day | ||||||||
| Olive oil | Lowest tertile | Greatest tertile | ||||||||
| Fish | <median servings/day | >median servings/day | Lowest tertile | Greatest tertile | ||||||
| Dairy products | Greatest tertile | Lowest tertile | ||||||||
| Low-fat dairy | Lowest quintile | Greatest quintile | ||||||||
| Sodium | Highest decile | Lowest decile (mg/day) | Greatest quintile | Lowest quintile | ||||||
| Alcohol | 0 or >2.5 servings/day | 0.5–1.5 servings/day | 0.5–1.5 drinks/day | <5 or <25 d/day | 5–25 d/day | |||||
| Sugar-sweetened beverages and fruit juices | ≥1 | 0 | ||||||||
| Total maximum score | 87.5 | 110 | 9 | 18 | 40 | |||||
| Median Value | AHEI | AHEI-2010 | aMED | rMED | DASH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (13–32) n = 70 | Q4 (48–78) n = 68 | p-value 1 | Q1 (27–56) n = 69 | Q4 (72–97) n = 68 | p-value | Q1 (0–3) n = 69 | Q4 (7–10) n = 55 | p-value | Q1 (2–9) n = 165 | Q4 (13–15) n = 27 | p-value | Q1 (11–19) n = 74 | Q4 (28–35) n = 61 | p-value 1 | |
| Age (years) | 27.5 (23.0; 32.0) | 31.0 (26.0; 34.8) | 0.07 | 28.0 (23.5; 32.0) | 33.0 (29.0; 35.0) | 0.00 | 27.0 (23.0; 32.0) | 30.0 (26.0; 34.0) | 0.01 | 29.0 (24.0;33.0) | 31.0 (26.0;34.0) | 0.43 | 31.0 (23.8; 35.0) | 28.0 (24.0; 33.0) | 0.25 |
| BMI (kg/m2) | 25.2 (21.4; 32.3) | 21.6 (20.1; 23.9) | 0.00 | 25.9 (20.5; 30.9) | 21.7 (20.1; 23.7) | 0.01 | 24.2 (20.9; 30.9) | 21.9 (20.6; 24.6) | 0.00 | 22.7 (20.1;27.5) | 22.0 (19.7;24.3) | 0.14 | 23.2 (20.8; 28.1) | 21.9 (20.4; 26.6) | 0.91 |
| Calories intake (Kcal) | 1537.5 (1230.6; 1972.7) | 2188.3 (1723.1; 2847.6) | 0.00 | 1908.1 (1448.5; 2310.5) | 1737.8 (1420.7; 2358.6) | 0.67 | 1456.9 (1178.5; 1937.7) | 2131.7 (1672.4; 2928.4) | 0.00 | 1945.3 (1605.1;2384.6) | 1466.2 (1288.2;1832.3) | 0.00 | 1338.0 (1086.8; 1570.6) | 2775.2 (2211.5; 3307.3) | 0.00 |
| Physical activity (h/week) | 3.6 (0.5; 11.9) | 8.4 (5.5; 14.0) | 0.00 | 5.0 (2.0; 14.0) | 8.0 (5.3; 14.3) | 0.00 | 4.7 (0.6; 10.8) | 8.0 (5.5; 13.8) | 0.00 | 6.0 (2.3;13.7) | 4.3 (0.0; 8.5) | 0.01 | 5.0 (0.8; 10.3) | 8.0 (3.1; 15.9) | 0.17 |
| Alcohol (g/day) | 0.4 (0.0; 1.4) | 3.6 (1.9; 6.3) | 0.00 | 0.5 (0.0; 1.7) | 3.6 (1.3; 6.6) | 0.00 | 0.9 (0.0; 2.4) | 5.8 (2.9; 8.1) | 0.00 | 1.3 (0.0;3.5) | 7.0 (3.8; 10.0) | 0.00 | 1.3 (0.5; 5.9) | 2.9 (0.6; 5.4) | 0.64 |
| Caffeine (mg/day) | 31.2 (8.3; 49.4) | 48.3 (18.5; 77.1) | 0.02 | 33.4 (13.5; 55.2) | 51.7 (22.4; 81.3) | 0.04 | 35.4 (13.2; 56.2) | 56.6 (22.0; 94.8) | 0.05 | 37.0 (14.7;68.4) | 46.3 (20.4; 99.0) | 0.21 | 37.3 (12.5; 67.3) | 47.3 (18.0; 79.9) | 0.46 |
| Carbohydrate (g/day) | 158.5 (131.4; 181.5) | 192.4 (170.9; 207.2) | 0.00 | 170.5 (137.3; 196.1) | 190.5 (167.8; 207.0) | 0.00 | 165.0 (132.8; 193.6) | 186.8 (164.5; 198.2) | 0.01 | 175.0 (149.7;200.4) | 167.2 (155.4; 193.5) | 0.83 | 163.4 (139.3; 189.1) | 185.3 (166.7; 204.0) | 0.00 |
| Saturated fats (g/day) | 22.1 (19.7; 25.3) | 17.9 (14.2; 21.4) | 0.00 | 22.5 (19.9; 26.0) | 16.6 (13.8; 19.7) | 0.00 | 21.3 (19.2; 24.9) | 18.8 (16.3; 22.1) | 0.00 | 21.2 (18.5;24.4) | 17.9 (15.6; 21.4) | 0.00 | 19.7 (16.2; 22.5) | 21.6 (19.3; 25.4) | 0.02 |
| Omega-3 (mg/day) | 1.3 (1.2; 1.4) | 1.7 (1.5; 2.2) | 0.00 | 1.3 (1.1; 1.5) | 1.6 (1.4; 2.0) | 0.00 | 1.2 (1.0; 1.4) | 1.7 (1.5; 2.2) | 0.00 | 1.5 (1.3;2.0) | 1.4 (1.1; 1.5) | 0.00 | 1.3 (1.1; 1.5) | 1.8 (1.5; 2.2) | 0.00 |
| Range for EachQuartile of Index 2 | Total PCOS | Anovulatory | Ovulatory | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases = 121 | Cases = 88 | Cases = 33 | ||||||||
| OR 1 | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | ||
| AHEI2010 | ||||||||||
| Q1 (27–56) | Ref. | Ref. | Ref. | |||||||
| Q2 (57–63) | 1.0 | (0.5; 2.0) | 0.93 | 1.1 | (0.5; 2.3) | 0.81 | 0.8 | (0.3; 2.3) | 0.69 | |
| Q3 (64–71) | 0.6 | (0.3; 1.2) | 0.14 | 0.5 | (0.2; 1.1) | 0.09 | 1.1 | (0.4; 2.9) | 0.89 | |
| Q4 (72–97) | 0.7 | (0.3; 1.6) | 0.44 | 0.9 | (0.4; 2.0) | 0.79 | 0.7 | (0.2; 2.1) | 0.50 | |
| p-value for trend | 0.41 | 0.24 | 0.84 | |||||||
| AHEI | ||||||||||
| Q1 (13–32) | Ref. | Ref. | Ref. | |||||||
| Q2 (33–40) | 1.6 | (0.8; 3.5) | 0.22 | 1.8 | (0.8; 3.9) | 0.17 | 1.0 | (0.4; 2.8) | 1.00 | |
| Q3 (41–47) | 1.0 | (0.5; 2.3) | 0.94 | 1.2 | (0.5; 2.8) | 0.66 | 0.8 | (0.2; 2.4) | 0.65 | |
| Q4 (48–78) | 0.8 | (0.3; 2.0) | 0.65 | 1.0 | (0.4; 2.6) | 0.97 | 0.7 | (0.2; 2.2) | 0.50 | |
| p-value for trend | 0.31 | 0.41 | 0.86 | |||||||
| aMED | ||||||||||
| Q1 (0–3) | Ref. | Ref. | Ref. | |||||||
| Q2 (4) | 0.6 | (0.3; 1.2) | 0.16 | 0.8 | (0.4; 1.7) | 0.58 | 0.5 | (0.2; 1.5) | 0.23 | |
| Q3 (5–6) | 0.9 | (0.4; 2.0) | 0.86 | 1.2 | (0.5; 2.6) | 0.70 | 0.7 | (0.2; 2.0) | 0.50 | |
| Q4 (7–10) | 0.8 | (0.4; 2.0) | 0.68 | 0.8 | (0.3; 2.1) | 0.71 | 1.0 | (0.3; 3.3) | 0.97 | |
| p-value for trend | 0.48 | 0.75 | 0.55 | |||||||
| rMED | ||||||||||
| Q1 (2–9) | Ref. | Ref. | Ref. | |||||||
| Q2 (10) | 0.9 | (0.4; 2.0) | 0.82 | 1.1 | (0.5; 2.5) | 0.82 | 0.7 | (0.2; 2.3) | 0.59 | |
| Q3 (11–12) | 0.6 | (0.3; 1.3) | 0.19 | 0.9 | (0.4; 2.0) | 0.74 | 0.3 | (0.1; 1.5) | 0.15 | |
| Q4 (13–15) | 1.6 | (0.7; 3.9) | 0.31 | 1.6 | (0.6; 4.1) | 0.33 | 1.1 | (0.3; 3.8) | 0.85 | |
| p-value for trend | 0.33 | 0.73 | 0.48 | |||||||
| DASH | ||||||||||
| Q1 (11–19) | Ref. | Ref. | Ref. | |||||||
| Q2 (20–23) | 0.8 | (0.4; 1.7) | 0.58 | 1.0 | (0.5; 2.3) | 0.98 | 0.6 | (0.2; 1.9) | 0.44 | |
| Q3 (24–27) | 1.1 | (0.5; 2.5) | 0.84 | 0.9 | (0.4; 2.3) | 0.90 | 1.3 | (0.4; 3.9) | 0.66 | |
| Q4 (28–35) | 1.6 | (0.5; 4.7) | 0.39 | 2.9 | (0.9; 9.3) | 0.07 | 0.4 | (0.1; 2.0) | 0.26 | |
| p-value for trend | 0.51 | 0.09 | 0.26 | |||||||
| Index | H + O + POM 2 | H + O 3 | H + POM 4 | O + POM 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases = 52 | Cases = 18 | Cases = 33 | Cases = 18 | ||||||||||
| OR 1 | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | ||
| AHEI-2010 | |||||||||||||
| Q1 (27–56) | Ref. | Ref. | Ref. | Ref. | |||||||||
| Q2 (57–63) | 1.5 | (0.7; 3.5) | 0.34 | 0.2 | (0.0; 0.7) | 0.01 | 0.8 | (0.3; 2.3) | 0.69 | 4.9 | (0.9; 25.6) | 0.06 | |
| Q3 (64–71) | 0.7 | (0.3; 1.8) | 0.43 | 0.1 | (0.0; 0.9) | 0.04 | 1.1 | (0.4; 2.9) | 0.89 | 2.5 | (0.4; 16.7) | 0.35 | |
| Q4 (72–97) | 1.1 | (0.4; 2.9) | 0.85 | 0.2 | (0.0; 1.2) | 0.08 | 0.7 | (0.2; 2.1) | 0.50 | 4.9 | (0.8; 30.4) | 0.09 | |
| p-value for trend | 0.42 | 0.02 | 0.84 | 0.24 | |||||||||
| AHEI | |||||||||||||
| Q1 (13–32) | Ref. | Ref. | Ref. | Ref. | |||||||||
| Q2 (33–40) | 2.3 | (0.9; 6.3) | 0.09 | 0.4 | (0.1; 1.5) | 0.17 | 1.0 | (0.4; 2.8) | 1.00 | 2.9 | (0.7; 12.9) | 0.15 | |
| Q3 (41–47) | 2.7 | (1.0; 7.3) | 0.06 | 0.0 | (0.0; −) | 1.00 | 0.8 | (0.2; 2.4) | 0.65 | 1.8 | (0.4; 8.8) | 0.48 | |
| Q4 (48–78) | 1.7 | (0.5; 5.5) | 0.38 | 0.3 | (0.0; 1.6) | 0.15 | 0.7 | (0.2; 2.2) | 0.50 | 1.5 | (0.2; 9.5) | 0.69 | |
| p-value for trend | 0.21 | 0.41 | 0.86 | 0.48 | |||||||||
| aMED | |||||||||||||
| Q1 (0–3) | Ref. | Ref. | Ref. | Ref. | |||||||||
| Q2 (4) | 1.5 | (0.6; 3.7) | 0.38 | 0.2 | (0.0; 0.9) | 0.04 | 0.5 | (0.2; 1.5) | 0.23 | 1.1 | (0.2; 5.8) | 0.90 | |
| Q3 (5–6) | 1.4 | (0.6; 3.7) | 0.46 | 0.3 | (0.1; 1.4) | 0.13 | 0.7 | (0.2; 2.0) | 0.50 | 3.6 | (0.8; 16.8) | 0.11 | |
| Q4 (7–10) | 1.1 | (0.4; 3.5) | 0.86 | 0.2 | (0.0; 1.8) | 0.15 | 1.0 | (0.3; 3.3) | 0.97 | 3.2 | (0.5; 18.7) | 0.20 | |
| p-value for trend | 0.78 | 0.12 | 0.55 | 0.28 | |||||||||
| rMED | |||||||||||||
| Q1 (2–9) | Ref. | Ref. | Ref. | Ref. | |||||||||
| Q2 (10) | 1.1 | (0.4; 2.9) | 0.86 | 0.7 | (0.1; 6.3) | 0.77 | 0.7 | (0.2;2.3) | 0.59 | 1.4 | (0.4; 5.6) | 0.63 | |
| Q3 (11–12) | 0.8 | (0.3; 2.1) | 0.60 | 1.5 | (0.4; 5.6) | 0.57 | 0.3 | (0.1; 1.5) | 0.15 | 0.5 | (0.1; 2.4) | 0.35 | |
| Q4 (13–15) | 1.9 | (0.6; 5.4) | 0.25 | 1.6 | (0.3; 9.1) | 0.61 | 1.1 | (0.3; 3.8) | 0.85 | 0.6 | (0.1; 4.9) | 0.60 | |
| p-value for trend | 0.60 | 0.88 | 0.48 | 0.68 | |||||||||
| Index | H + O + POM | H + O | H + POM | O + POM | |||||||||
| Cases = 52 | Cases = 18 | Cases = 33 | Cases = 18 | ||||||||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | ||
| DASH | |||||||||||||
| Q1 (11–19) | Ref. | Ref. | Ref. | Ref. | |||||||||
| Q2 (20–23) | 1.0 | (0.4; 2.6) | 0.96 | 1.1 | (0.3; 4.3) | 0.84 | 0.6 | (0.2; 1.9) | 0.44 | 0.8 | (0.1; 5.1) | 0.79 | |
| Q3 (24–27) | 0.8 | (0.2; 2.4) | 0.64 | 0.3 | (0.1; 2.3) | 0.27 | 1.3 | (0.4; 3.9) | 0.66 | 4.6 | (0.9; 24.4) | 0.08 | |
| Q4 (28–35) | 3.2 | (0.9; 11.9) | 0.08 | 0.0 | (0.0; −) | 1.00 | 0.4 | (0.1; 2.0) | 0.26 | 9.2 | (1.1; 74.7) | 0.04 | |
| p-value for trend | 0.07 | 0.84 | 0.26 | 0.05 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutillas-Tolín, A.; Arense-Gonzalo, J.J.; Mendiola, J.; Adoamnei, E.; Navarro-Lafuente, F.; Sánchez-Ferrer, M.L.; Prieto-Sánchez, M.T.; Carmona-Barnosi, A.; Vioque, J.; Torres-Cantero, A.M. Are Dietary Indices Associated with Polycystic Ovary Syndrome and Its Phenotypes? A Preliminary Study. Nutrients 2021, 13, 313. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020313
Cutillas-Tolín A, Arense-Gonzalo JJ, Mendiola J, Adoamnei E, Navarro-Lafuente F, Sánchez-Ferrer ML, Prieto-Sánchez MT, Carmona-Barnosi A, Vioque J, Torres-Cantero AM. Are Dietary Indices Associated with Polycystic Ovary Syndrome and Its Phenotypes? A Preliminary Study. Nutrients. 2021; 13(2):313. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020313
Chicago/Turabian StyleCutillas-Tolín, Ana, Julián Jesús Arense-Gonzalo, Jaime Mendiola, Evdochia Adoamnei, Fuensanta Navarro-Lafuente, María Luisa Sánchez-Ferrer, María Teresa Prieto-Sánchez, Ana Carmona-Barnosi, Jesús Vioque, and Alberto M. Torres-Cantero. 2021. "Are Dietary Indices Associated with Polycystic Ovary Syndrome and Its Phenotypes? A Preliminary Study" Nutrients 13, no. 2: 313. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020313