Increased Omega-3 Fatty Acid Intake Is Inversely Associated with Subclinical Inflammation in Healthy Elderly Men, Based on the 2015–2018 Korean National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions of High Cardiovascular Disease Risk Subclinical Inflammation and ω3FA Ratio
2.3. Definitions of Other Variables
2.4. Assessment of Daily Nutrient Intake
2.5. Statistical Analysis
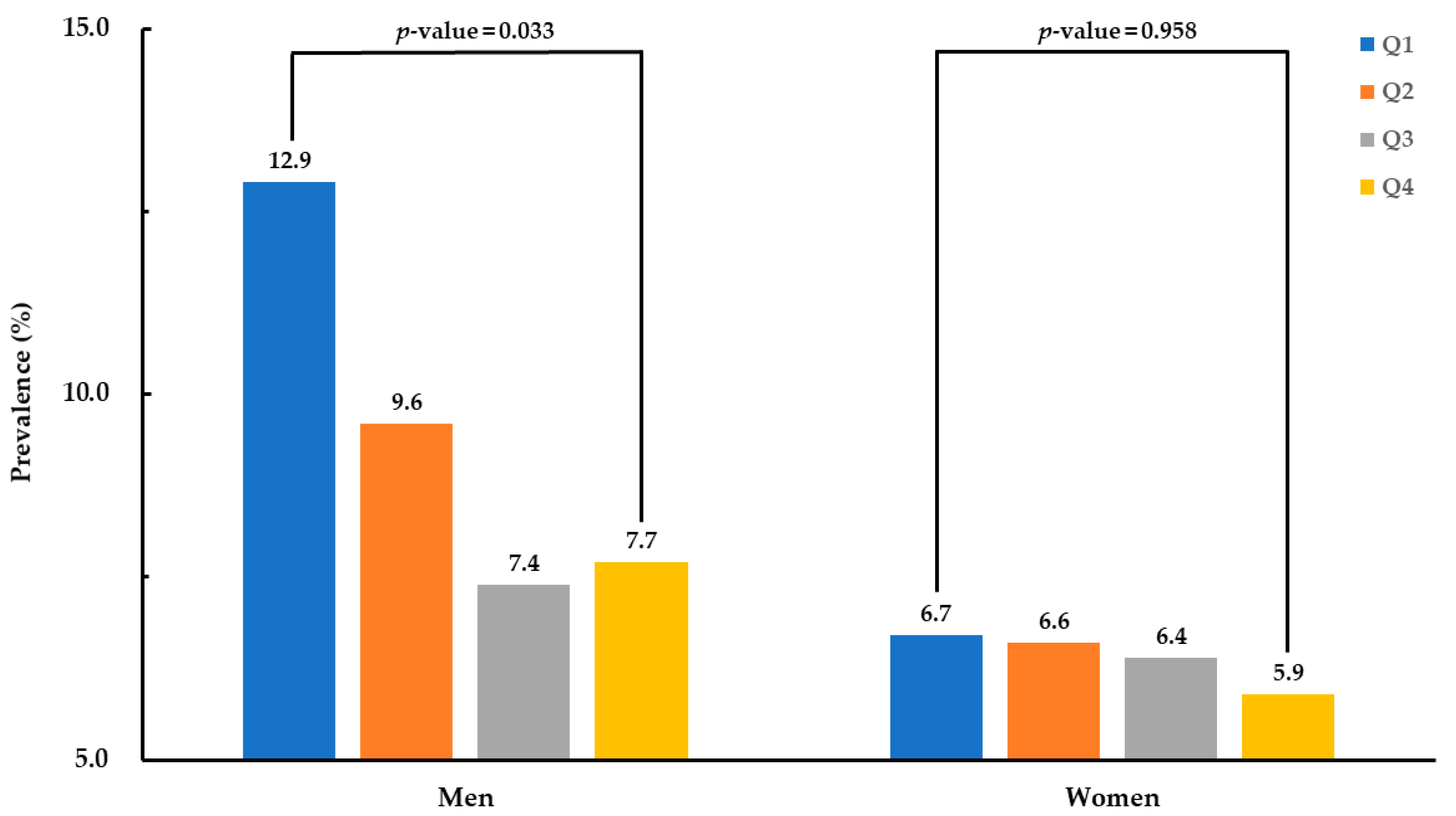
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Amore, P.J. Evolution of C-reactive protein as a cardiac risk factor. Lab. Med. 2005, 36, 234–238. [Google Scholar] [CrossRef]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) and hypertension: A review of vasodilatory mechanisms of DHA and EPA. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Siegel, G.; Ermilov, E. Omega-3 fatty acids: Benefits for cardio-cerebro-vascular diseases. Atherosclerosis 2012, 225, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association; Nutrition, C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III); National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2001. [Google Scholar]
- Kim, Y. The Korea national health and nutrition examination survey (KNHANES): Current status and challenges. Epidemiol. Health 2014, 36, e2014002. [Google Scholar] [CrossRef] [Green Version]
- Kweon, S.; Kim, Y.; Jang, M.-j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Lampón, N.; Hermida-Cadahia, E.F.; Riveiro, A.; Tutor, J.C. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann. Hepatol. 2012, 11, 356–363. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Statistics Korea. Causes of Death Statistics in 2018. Available online: http://kostat.go.kr/portal/eng/pressReleases/1/index.board?bmode=read&aSeq=378787 (accessed on 23 November 2020).
- American Heart Association. Heart Disease and Stroke Statistics—2003 Update; American Heart Association: Dallas, TX, USA, 2002. [Google Scholar]
- Calder, P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.C.; Moffatt, R.J.; Stamford, B.A. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis 2008, 201, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The cardiovascular therapeutic potential of propolis—A comprehensive review. Biology 2021, 10, 27. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Pasceri, V.; Willerson, J.T.; Yeh, E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000, 102, 2165–2168. [Google Scholar] [CrossRef]
- Verma, S.; Li, S.-H.; Badiwala, M.V.; Weisel, R.D.; Fedak, P.W.; Li, R.-K.; Dhillon, B.; Mickle, D.A. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002, 105, 1890–1896. [Google Scholar] [CrossRef] [Green Version]
- Abe, N.; Osanai, T.; Fujiwara, T.; Kameda, K.; Matsunaga, T.; Okumura, K. C-reactive protein-induced upregulation of extracellular matrix metalloproteinase inducer in macrophages: Inhibitory effect of fluvastatin. Life Sci. 2006, 78, 1021–1028. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Yang, X.-h.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goua, M.; Mulgrew, S.; Frank, J.; Rees, D.; Sneddon, A.; Wahle, K. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: Involvement of the transcription factor NF-κB? Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant-and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Qiu, Y.; Mu, Y.; Zhang, X.-J.; Liu, L.; Hou, X.-H.; Zhang, L.; Xu, X.-N.; Ji, A.-L.; Cao, R. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef]
- De Caterina, R.; Cybulsky, M.I.; Clinton, S.K.; Gimbrone, M.A., Jr.; Libby, P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Khalfoun, B.; Thibault, F.; Watier, H.; Bardos, P.; Lebranchu, Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv. Exp. Med. Biol. 1997, 400, 589. [Google Scholar]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127,477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Grewal, N.K.; Mosdøl, A.; Aunan, M.B.; Monsen, C.; Torheim, L.E. Development and pilot testing of 24-hour multiple-pass recall to assess dietary intake of toddlers of Somali-and Iraqi-born mothers living in Norway. Nutrients 2014, 6, 2333–2347. [Google Scholar] [CrossRef] [Green Version]
- Korea Disease Control and Prevention Agency. Guideline for Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII); Korea Disease Control and Prevention Agency: Osong, Korea, 2018. [Google Scholar]
| Characteristic | Males | Females | p-Value |
|---|---|---|---|
| Number | 2028 | 2776 | |
| Age, years | 69.3 ± 0.2 | 69.1 ± 0.2 | 0.549 |
| BMI, kg/m2 | 23.9 ± 0.1 | 24.3 ± 0.1 | <0.001 * |
| Energy intake, Kcal/day | 2041.7 ± 21.5 | 1556.0 ± 15.2 | <0.001 * |
| Carbohydrate intake, g/day | 333.3 ± 3.6 | 275.2 ± 2.8 | <0.001 * |
| Protein intake, g/day | 69.3 ± 0.9 | 51.3 ± 0.6 | <0.001 * |
| Fat intake, g/day | 34.5 ± 0.7 | 26.1 ± 0.5 | <0.001 * |
| ω3FA ratio, % | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.661 |
| Omega-6/omega-3 ratio | 6.3 ± 0.2 | 6.4 ± 0.1 | 0.807 |
| hsCRP, mg/L | 1.2 ± 0.0 | 1.0 ± 0.0 | <0.001 * |
| Total cholesterol, mg/dL | 187.4 ± 1.0 | 199.6 ± 0.9 | <0.001 * |
| Systolic blood pressure, mmHg | 126.9 ± 0.4 | 127.7 ± 0.4 | 0.157 |
| Fasting plasma glucose, mg/dL | 102.9 ± 0.5 | 99.8 ± 0.3 | <0.001 * |
| AST, IU/L | 24.8 ± 0.3 | 23.6 ± 0.2 | 0.002 * |
| Current smoking, % | 22.3 ± 1.1 | 2.7 ± 0.4 | <0.001 * |
| Heavy alcohol intake, % | 12.7 ± 0.9 | 1.2 ± 0.2 | <0.001 * |
| Economic status, % | <0.001 * | ||
| Low | 30.4 ± 1.3 | 38.9 ± 1.2 | |
| Middle–low | 28.3 ± 1.1 | 27.2 ± 1.0 | |
| Middle–high | 22.3 ± 1.1 | 19.3 ± 1.0 | |
| High | 19.1 ± 1.1 | 14.6 ± 1.0 | |
| Marital status, % | <0.001 * | ||
| Married and not separated | 89.7 ± 0.8 | 58.7 ± 1.2 | |
| Single | 10.3 ± 0.8 | 41.3 ± 1.2 | |
| Education duration, % | <0.001 * | ||
| <6 years | 34.7 ± 1.4 | 58.6 ± 1.3 | |
| 6–<9 years | 18.2 ± 1.2 | 16.5 ± 0.8 | |
| 9–<12 years | 26.2 ± 1.2 | 16.6 ± 0.9 | |
| ≥12 years | 20.9 ± 1.2 | 8.2 ± 0.7 | |
| Occupation, % | <0.001 * | ||
| Office workers | 9.7 ± 0.8 | 2.4 ± 0.4 | |
| Manual workers | 42.3 ± 1.5 | 32.5 ± 1.1 | |
| Other | 48.1 ± 1.4 | 65.1 ± 1.1 | |
| Sufficient physical activity, % | 41.9 ± 1.4 | 34.8 ± 1.2 | <0.001 * |
| Males | Q1 | Q2 | Q3 | Q4 | p-Value |
|---|---|---|---|---|---|
| (<0.3%) | (0.3%–<0.6%) | (0.6%–<1.0%) | (≥1.0%) | ||
| Number | 538 | 498 | 490 | 502 | |
| Age, years | 70.4 ± 0.4 | 68.8 ± 0.3 | 69.2 ± 0.4 | 68.7 ± 0.3 | 0.001 * |
| BMI, kg/m2 | 23.8 ± 0.2 | 23.7 ± 0.1 | 23.9 ± 0.1 | 24.0 ± 0.1 | 0.315 |
| Energy intake, Kcal/day | 1873.6 ± 43.1 | 2093.2 ± 36.6 | 2090.1 ± 41.9 | 2109.8 ± 38.6 | <0.001 * |
| Carbohydrate intake, g/day | 318.0 ± 6.9 | 352.6 ± 6.3 | 335.5 ± 7.0 | 326.9 ± 5.7 | 0.001 * |
| Protein intake, g/day | 54.5 ± 1.5 | 69.2 ± 1.5 | 74.1 ± 1.6 | 79.3 ± 1.8 | <0.001 * |
| Fat intake, g/day | 24.2 ± 1.1 | 33.1 ± 1.2 | 36.1 ± 1.2 | 44.4 ± 1.5 | <0.001 * |
| ω3FA ratio, % | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 1.9 ± 0.1 | <0.001 * |
| Omega-6/omega-3 ratio | 10.7 ± 0.6 | 6.7 ± 0.2 | 4.9 ± 0.1 | 3.0 ± 0.1 | <0.001 * |
| hsCRP, mg/L | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.024 * |
| Total cholesterol, mg/dL | 190.5 ± 1.9 | 186.1 ± 1.7 | 187.1 ± 1.8 | 186.1 ± 1.9 | 0.301 |
| Systolic blood pressure, mmHg | 128.7 ± 0.8 | 128.3 ± 0.8 | 125.9 ± 0.9 | 124.5 ± 0.8 | 0.001 * |
| Fasting plasma glucose, mg/dL | 103.8 ± 1.0 | 103.7 ± 1.1 | 102.3 ± 1.1 | 101.8 ± 0.8 | 0.273 |
| AST, IU/L | 25.9 ± 0.6 | 24.1 ± 0.4 | 24.2 ± 0.4 | 25.0 ± 0.9 | 0.045 * |
| Current smoking, % | 27.1 ± 2.2 | 24.0 ± 2.3 | 20.4 ± 2.2 | 17.6 ± 1.8 | 0.011 * |
| Heavy alcohol intake, % | 16.0 ± 1.7 | 11.5 ± 1.7 | 13.0 ± 1.8 | 10.4 ± 1.5 | 0.077 |
| Economic status, % | <0.001 * | ||||
| Low | 42.5 ± 2.4 | 29.7 ± 2.5 | 26.8 ± 2.3 | 22.5 ± 2.1 | |
| Middle–low | 27.1 ± 2.2 | 27.7 ± 2.2 | 26.4 ± 2.1 | 31.9 ± 2.4 | |
| Middle–high | 17.0 ± 1.9 | 21.8 ± 2.1 | 24.6 ± 2.1 | 25.6 ± 2.3 | |
| High | 13.4 ± 1.8 | 20.8 ± 1.9 | 22.2 ± 2.3 | 20.0 ± 2.0 | |
| Marital status, % | 0.001 * | ||||
| Married and not separated | 85.0 ± 1.8 | 88.6 ± 1.7 | 91.8 ± 1.5 | 93.2 ± 1.3 | |
| Single | 15.0 ± 1.8 | 11.4 ± 1.7 | 8.2 ± 1.5 | 6.8 ± 1.3 | |
| Education duration, % | <0.001 * | ||||
| <6 years | 48.2 ± 2.7 | 36.7 ± 2.6 | 29.4 ± 2.2 | 24.9 ± 2.5 | |
| 6–<9 years | 20.5 ± 2.2 | 17.7 ± 1.9 | 16.8 ± 1.9 | 17.7 ± 2.3 | |
| 9–<12 years | 18.7 ± 2.0 | 25.0 ± 2.2 | 28.6 ± 2.4 | 32.3 ± 2.6 | |
| ≥12 years | 12.7 ± 1.8 | 20.6 ± 2.1 | 25.1 ± 2.4 | 25.1 ± 2.2 | |
| Occupation, % | 0.471 | ||||
| Office workers | 6.0 ± 1.1 | 10.7 ± 1.5 | 10.9 ± 1.7 | 10.9 ± 1.5 | |
| Manual workers | 46.1 ± 2.7 | 44.3 ± 2.6 | 39.6 ± 2.8 | 39.3 ± 2.5 | |
| Other | 47.9 ± 2.6 | 45.1 ± 2.7 | 49.5 ± 2.9 | 49.7 ± 2.6 | |
| Sufficient physical activity, % | 39.5 ± 2.7 | 44.1 ± 2.5 | 41.6 ± 2.8 | 42.4 ± 2.7 | 0.675 |
| Females | Q1 | Q2 | Q3 | Q4 | p-Value |
| (<0.3%) | (0.3%–<0.6%) | (0.6%–<1.0%) | (≥1.0%) | ||
| Number | 710 | 695 | 682 | 689 | |
| Age, years | 70.7 ± 0.3 | 69.4 ± 0.3 | 68.7 ± 0.3 | 67.7 ± 0.3 | <0.001 * |
| BMI, kg/m2 | 24.4 ± 0.1 | 24.3 ± 0.1 | 24.1 ± 0.1 | 24.1 ± 0.1 | 0.302 |
| Energy intake, Kcal/day | 1438.4 ± 30.0 | 1546.2 ± 28.6 | 1572.1 ± 28.0 | 1667.2 ± 28.9 | <0.001 * |
| Carbohydrate intake, g/day | 277.8 ± 5.9 | 281.0 ± 5.3 | 271.9 ± 5.0 | 270.0 ± 4.8 | 0.388 |
| Protein intake, g/day | 40.0 ± 1.1 | 49.3 ± 1.0 | 55.1 ± 1.1 | 60.8 ± 1.3 | <0.001 * |
| Fat intake, g/day | 16.1 ± 0.7 | 22.7 ± 0.7 | 28.2 ± 0.8 | 37.4 ± 1.1 | <0.001 * |
| ω3FA ratio, % | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 | 1.9 ± 0.0 | <0.001 * |
| Omega-6/omega-3 ratio | 10.4 ± 0.4 | 6.6 ± 0.1 | 5.3 ± 0.1 | 3.2 ± 0.1 | <0.001 * |
| hsCRP, mg/L | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.251 |
| Total cholesterol, mg/dL | 198.7 ± 1.7 | 199.4 ± 1.7 | 198.4 ± 1.7 | 201.9 ± 1.8 | 0.507 |
| Systolic blood pressure, mmHg | 129.2 ± 0.9 | 128.1 ± 0.8 | 127.2 ± 0.8 | 126.2 ± 0.8 | 0.074 |
| Fasting plasma glucose, mg/dL | 101.6 ± 0.8 | 98.6 ± 0.6 | 99.9 ± 0.7 | 99.0 ± 0.6 | 0.012 * |
| AST, IU/L | 23.6 ± 0.4 | 23.7 ± 0.4 | 23.8 ± 0.6 | 23.3 ± 0.3 | 0.851 |
| Current smoking, % | 2.3 ± 0.6 | 3.8 ± 1.0 | 1.8 ± 0.5 | 3.0 ± 0.9 | 0.298 |
| Heavy alcohol intake, % | 0.8 ± 0.3 | 1.6 ± 0.6 | 1.1 ± 0.4 | 1.2 ± 0.4 | 0.737 |
| Economic status, % | <0.001 * | ||||
| Low | 49.1 ± 2.3 | 40.0 ± 2.2 | 34.3 ± 2.3 | 32.0 ± 2.1 | |
| Middle–low | 27.9 ± 2.1 | 26.1 ± 1.9 | 25.1 ± 2.0 | 29.5 ± 2.0 | |
| Middle–high | 15.7 ± 1.9 | 19.7 ± 1.8 | 20.9 ± 2.0 | 21.0 ± 1.8 | |
| High | 7.3 ± 1.1 | 14.2 ± 1.7 | 19.6 ± 2.0 | 17.5 ± 1.8 | |
| Marital status, % | <0.001 * | ||||
| Married and not separated | 50.7 ± 2.3 | 57.5 ± 2.1 | 60.3 ± 2.3 | 66.3 ± 2.2 | |
| Single | 49.3 ± 2.3 | 42.5 ± 2.1 | 40.0 ± 2.3 | 33.7 ± 2.2 | |
| Education duration, % | <0.001 * | ||||
| <6 years | 72.3 ± 2.2 | 61.6 ± 2.2 | 51.8 ± 2.3 | 49.2 ± 2.3 | |
| 6–<9 years | 13.5 ± 1.6 | 15.8 ± 1.6 | 18.0 ± 1.6 | 18.7 ± 1.8 | |
| 9–<12 years | 10.2 ± 1.5 | 15.6 ± 1.7 | 20.7 ± 2.0 | 19.7 ± 1.9 | |
| ≥12 years | 4.0 ± 1.0 | 7.0 ± 1.1 | 9.5 ± 1.4 | 12.3 ± 1.6 | |
| Occupation, % | 0.996 | ||||
| Office workers | 0.9 ± 0.4 | 1.5 ± 0.7 | 2.9 ± 0.7 | 4.2 ± 0.9 | |
| Manual workers | 35.2 ± 2.3 | 34.8 ± 2.2 | 31.2 ± 2.0 | 29.1 ± 1.9 | |
| Other | 63.9 ± 2.3 | 63.7 ± 2.3 | 65.9 ± 2.1 | 66.7 ± 2.0 | |
| Sufficient physical activity, % | 30.3 ± 2.2 | 36.5 ± 2.2 | 35.5 ± 2.2 | 36.8 ± 2.3 | 0.122 |
| Ratio of Daily Total Fat and Fatty Acids Intake to Energy Intake (%) | Subclinical Inflammation | No Subclinical Inflammation | p-Value |
|---|---|---|---|
| Males, Number | 197 | 1831 | |
| Total fat | 13.7 ± 0.6 | 14.6 ± 0.2 | 0.129 |
| SFA | 4.3 ± 0.2 | 4.4 ± 0.1 | 0.639 |
| MUFA | 4.1 ± 0.2 | 4.4 ± 0.1 | 0.282 |
| PUFA | 3.6 ± 0.2 | 4.2 ± 0.1 | 0.001 * |
| Omega-3 FA | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.001 * |
| Omega-6 FA | 3.0 ± 0.1 | 3.4 ± 0.1 | 0.004 * |
| Omega-6/omega-3 ratio † | 6.8 ± 0.4 | 6.3 ± 0.2 | 0.229 |
| Females, Number | 184 | 2592 | |
| Total fat | 13.6 ± 0.7 | 14.5 ± 0.2 | 0.241 |
| SFA | 4.3 ± 0.2 | 4.4 ± 0.1 | 0.675 |
| MUFA | 4.1 ± 0.3 | 4.3 ± 0.1 | 0.430 |
| PUFA | 3.7 ± 0.2 | 4.3 ± 0.1 | 0.011 * |
| Omega-3 FA | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.491 |
| Omega-6 FA | 3.0 ± 0.1 | 3.4 ± 0.1 | 0.005 * |
| Omega-6/omega-3 ratio † | 6.2 ± 0.4 | 6.4 ± 0.1 | 0.752 |
| Males | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| (<0.3%) | (0.3%–<0.6%) | (0.6%–<1.0%) | (≥1.0%) | |
| Number | 538 | 498 | 490 | 502 |
| Model 1 | 1 (ref) | 0.754 (0.489–1.162) | 0.561 (0.356–0.883) | 0.595 (0.368–0.962) |
| Model 2 | 1 (ref) | 0.752 (0.487–1.161) | 0.555 (0.353–0.873) | 0.590 (0.363–0.958) |
| Model 3 | 1 (ref) | 0.740 (0.465–1.177) | 0.564 (0.341–0.930) | 0.549 (0.317–0.953) |
| Females | Q1 | Q2 | Q3 | Q4 |
| (<0.3%) | (0.3%–<0.6%) | (0.6%–<1.0%) | (≥1.0%) | |
| Number | 710 | 695 | 682 | 689 |
| Model 1 | 1 (ref) | 1.030 (0.637–1.666) | 1.024 (0.620–1.694) | 0.972 (0.600–1.575) |
| Model 2 | 1 (ref) | 1.096 (0.668–1.797) | 1.094 (0.655–1.827) | 1.051 (0.642–1.719) |
| Model 3 | 1 (ref) | 1.066 (0.653–1.741) | 1.105 (0.600–1.718) | 0.934 (0.556–1.571) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Lee, J.H.; Lee, J.-w.; Kim, Y.; Kim, Y.-S.; You, H.-S.; Kang, H.-T. Increased Omega-3 Fatty Acid Intake Is Inversely Associated with Subclinical Inflammation in Healthy Elderly Men, Based on the 2015–2018 Korean National Health and Nutrition Examination Survey. Nutrients 2021, 13, 338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020338
Yang W, Lee JH, Lee J-w, Kim Y, Kim Y-S, You H-S, Kang H-T. Increased Omega-3 Fatty Acid Intake Is Inversely Associated with Subclinical Inflammation in Healthy Elderly Men, Based on the 2015–2018 Korean National Health and Nutrition Examination Survey. Nutrients. 2021; 13(2):338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020338
Chicago/Turabian StyleYang, Woojung, Jong Hun Lee, Jae-woo Lee, Yonghwan Kim, Ye-Seul Kim, Hyo-Sun You, and Hee-Taik Kang. 2021. "Increased Omega-3 Fatty Acid Intake Is Inversely Associated with Subclinical Inflammation in Healthy Elderly Men, Based on the 2015–2018 Korean National Health and Nutrition Examination Survey" Nutrients 13, no. 2: 338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020338






