Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
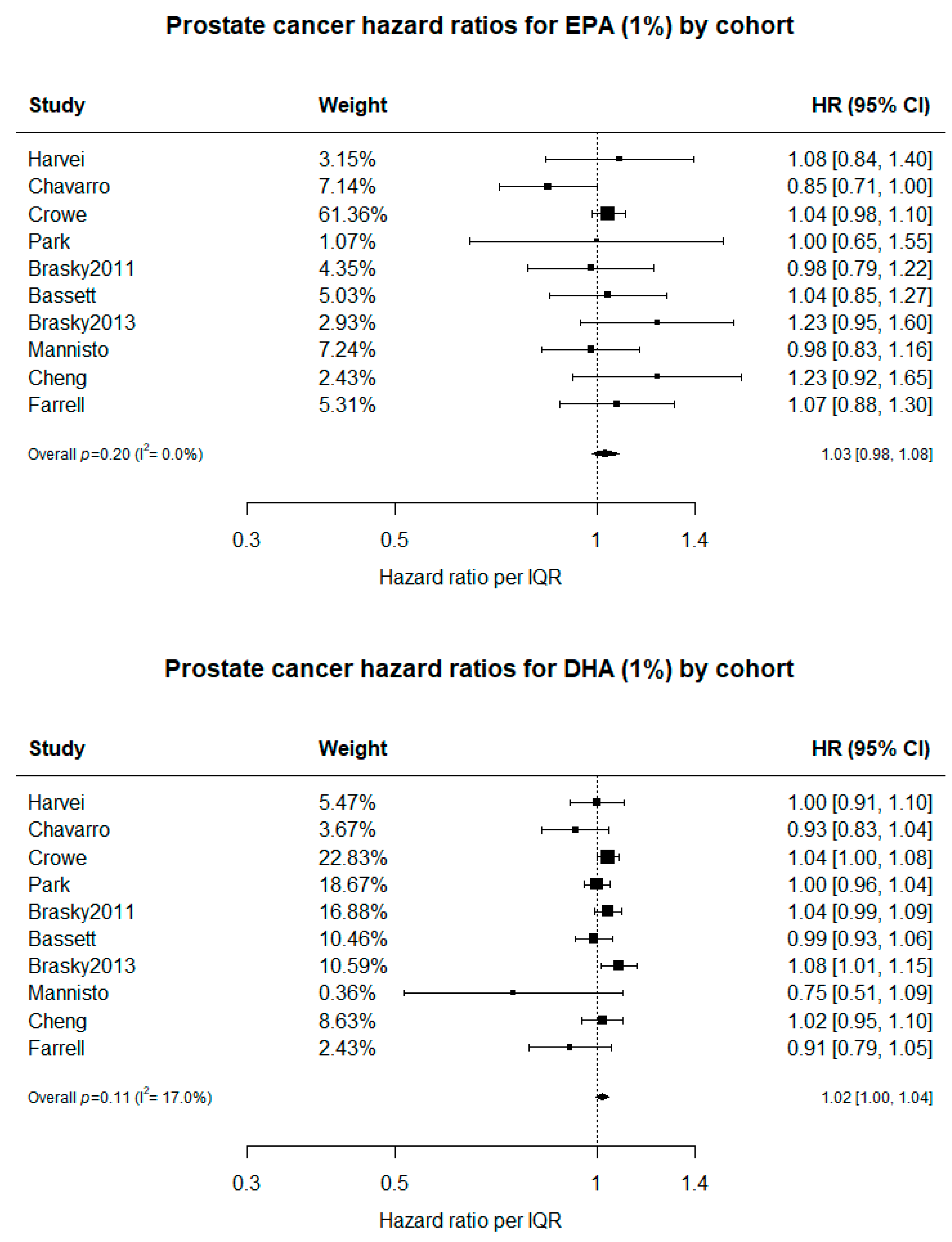
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urquiza-Salvat, N.; Pascual-Geler, M.; Lopez-Guarnido, O.; Rodrigo, L.; Martinez-Burgos, A.; Cozar, J.M.; Ocana-Peinado, F.M.; Alvarez-Cubero, M.J.; Rivas, A. Adherence to Mediterranean diet and risk of prostate cancer. Aging Male 2019, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.R. Diet and prostate cancer prevention. World J. Urol. 2012, 30, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Discacciati, A.; Orsini, N.; Wolk, A. Body mass index and incidence of localized and advanced prostate cancer—A dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Suburu, J.; Lim, K.; Calviello, G.; Chen, Y.Q. RE: Serum phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2014, 106, dju023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S.; Davidson, M.H. RE: Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2014, 106, dju019. [Google Scholar] [CrossRef] [Green Version]
- Brenna, J.T.; Burdge, G.C.; Crawford, M.A.; Clayton, P.; Cunnane, S.C.; Gow, R.; Hibbeln, J.R.; Sinclair, A.J.; Stein, J.; Willatts, P. RE: Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2014, 106, dju015. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Barnett, M.; Brasky, T.M.; Bueno-de-Mesquita, H.B.; Chajes, V.; Chavarro, J.E.; Chirlaque, M.D.; English, D.R.; et al. Circulating fatty acids and prostate cancer risk: Individual participant meta-analysis of prospective studies. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Q.; Zheng, J.S.; Yang, B.; Li, D. Effect of individual omega-3 fatty acids on the risk of prostate cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Epidemiol. 2015, 25, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aucoin, M.; Cooley, K.; Knee, C.; Fritz, H.; Balneaves, L.G.; Breau, R.; Fergusson, D.; Skidmore, B.; Wong, R.; Seely, D. Fish-Derived Omega-3 Fatty Acids and Prostate Cancer: A Systematic Review. Integr. Cancer Ther. 2017, 16, 32–62. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von, S.C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- DeFina, L.F.; Bassett, M.H.; Finley, C.E.; Barlow, C.E.; Willis, B.L.; Cooper, T.; Clark, S.M.; Harris, W.S.; Radford, N.B. Association between omega-3 fatty acids and serum prostate-specific antigen. Nutr. Cancer 2016, 68, 58–62. [Google Scholar] [CrossRef]
- Harris, W.S.; Pottala, J.V.; Vasan, R.S.; Larson, M.G.; Robins, S.J. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J. Nutr. 2012, 142, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 2007, 55, 217–223. [Google Scholar] [CrossRef] [Green Version]
- American_Cancer_Society. Prostate Cancer Risk Factors. Available online: https://www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/risk-factors.html (accessed on 11 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef]
- Moussa, H.; Nguile-Makao, M.; Robitaille, K.; Guertin, M.H.; Allaire, J.; Pelletier, J.F.; Moreel, X.; Gevariya, N.; Diorio, C.; Desmeules, P.; et al. Omega-3 Fatty Acids Survey in Men under Active Surveillance for Prostate Cancer: From Intake to Prostate Tissue Level. Nutrients 2019, 11, 1616. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Julin, B.; Glynn, A.; Hogberg, J.; Berglund, M.; Johansson, J.E.; Andersson, S.O.; Andren, O.; Giovannucci, E.; Wolk, A.; et al. Exposure to polychlorinated biphenyls and prostate cancer: Population-based prospective cohort and experimental studies. Carcinogenesis 2016, 37, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Risk-based consumption advice for farmed Atlantic and wild Pacific salmon contaminated with dioxins and dioxin-like compounds. Environ. Health Perspect. 2005, 113, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azordegan, N.; Fraser, V.; Le, K.; Hillyer, L.M.; Ma, D.W.; Fischer, G.; Moghadasian, M.H. Carcinogenesis alters fatty acid profile in breast tissue. Mol. Cell Biochem. 2013, 374, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pender-Cudlip, M.C.; Krag, K.J.; Martini, D.; Yu, J.; Guidi, A.; Skinner, S.S.; Zhang, Y.; Qu, X.; He, C.; Xu, Y.; et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013, 104, 760–764. [Google Scholar] [CrossRef]
- Blair, S.N.; Goodyear, N.N.; Gibbons, L.W.; Cooper, K.H. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA 1984, 252, 487–490. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djousse, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Lifestyle; et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [Green Version]
| Variable | Value |
|---|---|
| Age (years) | 53.4 (8.4) |
| White (332 missing) (%) | 4929 (93.4%) |
| Omega-3 Index (%) | 6.0 (1.9) |
| BMI (kg/m2) | 27.7 (3.9) |
| Maximal METS (674 missing) | 11.5 (2.1) |
| Glucose (mmol/L) | 5.4 (0.8) |
| Total cholesterol (mmol/L) | 4.8 (1.0) |
| HDL cholesterol (mmol/L) | 1.4 (0.4) |
| Triglycerides (mmol/L) | 1.3 (0.9) |
| Systolic BP (mmHg) | 120.8 (12.0) |
| Diastolic BP (mmHg) | 79.8 (8.7) |
| Prostate-specific antigen (ng/mL) | 1.1 (0.7) |
| Alcohol (drinks/week) | 6.7 (6.7) |
| Current Smoking, n (%) | 537 (9.6%) |
| Omega-3 supplement use (369 missing), n (%) | 2107 (40.2%) |
| Modeling Age of Diagnosis | Modeling Time to Diagnosis | |||||
|---|---|---|---|---|---|---|
| Omega-3 Index | HR (95% CI) | Adjusted HR 1 (95% CI) | Unadjusted HR (95% CI) | Age-Adjusted HR (95% CI) | Adjusted HR 2 (95% CI) | |
| Quintile | Q1: O3I < 4.4 | 1 | 1 | 1 | 1 | 1 |
| Q2: 4.4 ≤ O3I <5.3 | 0.54 (0.27,1.07) | 0.52 (0.26,1.04) | 0.64 (0.32,1.28) | 0.57 (0.29,1.14) | 0.54 (0.27,1.08) | |
| Q3: 5.3 ≤ O3I <6.3 | 0.72 (0.39,1.30) | 0.72 (0.39,1.31) | 0.94 (0.52,1.71) | 0.78 (0.43,1.42) | 0.79 (0.43,1.44) | |
| Q4: 6.3 ≤ O3I <7.6 | 0.67 (0.37,1.21) | 0.64 (0.36,1.16) | 0.99 (0.55,1.78) | 0.75 (0.42,1.36) | 0.71 (0.39,1.29) | |
| Q5: 7.6 ≥ O3I | 0.61 (0.34,1.07) | 0.55 (0.31,0.98) | 1.17 (0.67,2.07) | 0.73 (0.41,1.30) | 0.65 (0.36,1.16) | |
| P for trend | 0.2 | 0.13 | 0.25 | 0.6 | 0.36 | |
| Category | O3I < 4% | 1 | 1 | 1 | 1 | 1 |
| O3I 4–8% | 0.73 (0.41,1.30) | 0.70 (0.39,1.26) | 1.02 (0.57,1.83) | 0.81 (0.45,1.46) | 0.78 (0.44,1.41) | |
| O3I > 8% | 0.65 (0.33,1.27) | 0.60 (0.30,1.19) | 1.31 (0.67,2.58) | 0.79 (0.4,1.56) | 0.72 (0.36,1.43) | |
| P for trend | 0.2 | 0.17 | 0.2 | 0.23 | 0.38 | |
| Continuous | (per 1%) | 0.95 (0.86,1.04) | 0.93 (0.85,1.03) | 1.06 (0.97,1.17) | 0.98 (0.89,1.07) | 0.96 (0.87,1.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, S.W.; DeFina, L.F.; Tintle, N.L.; Leonard, D.; Cooper, K.H.; Barlow, C.E.; Haskell, W.L.; Pavlovic, A.; Harris, W.S. Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study. Nutrients 2021, 13, 384. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020384
Farrell SW, DeFina LF, Tintle NL, Leonard D, Cooper KH, Barlow CE, Haskell WL, Pavlovic A, Harris WS. Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study. Nutrients. 2021; 13(2):384. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020384
Chicago/Turabian StyleFarrell, Stephen W., Laura F. DeFina, Nathan L. Tintle, David Leonard, Kenneth H. Cooper, Carolyn E. Barlow, William L. Haskell, Andjelka Pavlovic, and William S. Harris. 2021. "Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study" Nutrients 13, no. 2: 384. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020384






