Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health
Abstract
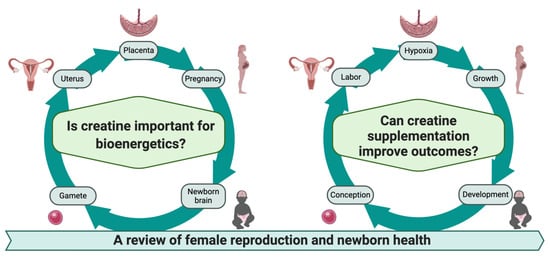
:1. Introduction
2. Creatine Metabolism in the Female Reproductive System
2.1. Oocytes and Surrounding Cells
2.2. Follicular Fluid, the Oviduct and Oviductal Fluid
2.3. The Endometrium
2.4. The Myometrium
3. Creatine Metabolism in the Human Placenta
4. Maternal Creatine Metabolism during Pregnancy
5. Fetal Creatine Metabolism and Use of Supplementary Creatine to Prevent Perinatal Brain Injury
6. Creatine Metabolism in the Neonate, with a Focus on the Potential Consequences of Preterm Birth
7. Conclusions and Research Road Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef]
- Canonaco, F.; Schlattner, U.; Pruett, P.S.; Wallimann, T.; Sauer, U. Functional expression of phosphagen kinase systems confers resistance to transient stresses in Saccharomyces cerevisiae by buffering the ATP pool. J. Biol. Chem. 2002, 277, 31303–31309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281 Pt 1, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.; Brosnan, M. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, E.; Chan, S.; Monfared, M.; Wallimann, T.; Clarke, K.; Neubauer, S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E399–E406. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Murphy, R.M. Creatine and the creatine transporter: A review. Mol. Cell. Biochem. 2001, 224, 169–181. [Google Scholar] [CrossRef]
- Schlattner, U.; Klaus, A.; Rios, S.R.; Guzun, R.; Kay, L.; Tokarska-Schlattner, M. Cellular compartmentation of energy metabolism: Creatine kinase microcompartments and recruitment of B-type creatine kinase to specific subcellular sites. Amino Acids 2016, 48, 1751–1774. [Google Scholar] [CrossRef]
- Umehara, T.; Kawai, T.; Goto, M.; Richards, J.S.; Shimada, M. Creatine enhances the duration of sperm capacitation: A novel factor for improving in vitro fertilization with small numbers of sperm. Hum. Reprod. 2018, 33, 1117–1129. [Google Scholar] [CrossRef] [Green Version]
- Mellanby, E. The metabolism of lactating women. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1913, 86, 88–109. [Google Scholar]
- Kao, L.; Tulac, S.; Lobo, S.a.; Imani, B.; Yang, J.; Germeyer, A.; Osteen, K.; Taylor, R.; Lessey, B.; Giudice, L. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002, 143, 2119–2138. [Google Scholar] [CrossRef] [PubMed]
- Ellery, S.J.; LaRosa, D.A.; Kett, M.M.; Della Gatta, P.A.; Snow, R.J.; Walker, D.W.; Dickinson, H. Maternal creatine homeostasis is altered during gestation in the spiny mouse: Is this a metabolic adaptation to pregnancy? BMC Pregnancy Childbirth 2015, 15, 92. [Google Scholar] [CrossRef] [Green Version]
- Ellery, S.J.; Murthi, P.; Davies-Tuck, M.L.; Gatta, P.D.; May, A.K.; Kowalski, G.M.; Callahan, D.L.; Bruce, C.R.; Alers, N.O.; Miller, S.L. Placental Creatine Metabolism in Cases of Placental Insufficiency and Reduced Fetal Growth. Mol. Hum. Reprod. 2019, 25, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ellery, S.J.; Murthi, P.; Gatta, P.A.D.; May, A.K.; Davies-Tuck, M.L.; Kowalski, G.M.; Callahan, D.L.; Bruce, C.R.; Wallace, E.M.; Walker, D.W. The Effects of Early-Onset Pre-Eclampsia on Placental Creatine Metabolism in the Third Trimester. Int. J. Mol. Sci. 2020, 21, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warzych, E.; Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. J. Reprod. Dev. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, M.M.-Y.; Manchester, J.K.; Yang, V.C.; Curato, A.D.; Strickler, R.C.; Lowry, O.H. Contrast in levels of metabolic enzymes in human and mouse ova. Biol. Reprod. 1988, 39, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, M.R.; Iyengar, C.W.L.; Chen, H.Y.; Brinster, R.L.; Bornslaeger, E.; Schultz, R.M. Expression of creatine kinase isoenzyme during oogenesis and embryogenesis in the mouse. Dev. Biol. 1983, 96, 263–268. [Google Scholar] [CrossRef]
- Forsey, K.E.; Ellis, P.J.; Sargent, C.A.; Sturmey, R.G.; Leese, H.J. Expression and localization of creatine kinase in the preimplantation embryo. Mol. Reprod. Dev. 2013, 80, 185–192. [Google Scholar] [CrossRef]
- Scantland, S.; Tessaro, I.; Macabelli, C.H.; Macaulay, A.D.; Cagnone, G.; Fournier, É.; Luciano, A.M.; Robert, C. The adenosine salvage pathway as an alternative to mitochondrial production of ATP in maturing mammalian oocytes. Biol. Reprod. 2014, 91, 75. [Google Scholar] [CrossRef]
- Fezai, M.; Warsi, J.; Lang, F. Regulation of the Na+, Cl-Coupled Creatine Transporter CreaT (SLC6A8) by the Janus Kinase JAK3. Neurosignals 2015, 23, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, J.-H.; Chae, Y.-J.; Ogawa, H.; Lee, M.-H.; Gerton, G.L. Creatine synthesis and transport systems in the male rat reproductive tract. Biol. Reprod. 1998, 58, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Liu, C.-H.; Lee, T.-H.; Wu, H.-M.; Huang, C.-C.; Huang, L.-S.; Chen, C.-M.; Cheng, E.-H. Association of creatin kinase B and peroxiredoxin 2 expression with age and embryo quality in cumulus cells. J. Assist. Reprod. Genet. 2010, 27, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhde, K.; van Tol, H.T.; Stout, T.A.; Roelen, B.A. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci. Rep. 2018, 8, 9477. [Google Scholar] [CrossRef] [PubMed]
- Fakih, H.; MacLusky, N.; DeCherney, A.; Wallimann, T.; Huszar, G. Enhancement of human sperm motility and velocity in vitro: Effects of calcium and creatine phosphate. Fertil. Steril. 1986, 46, 938–944. [Google Scholar] [CrossRef]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef]
- Edwards, R. Follicular fluid. Reproduction 1974, 37, 189–219. [Google Scholar] [CrossRef] [Green Version]
- Huyser, C.; Fourie, F.l.R.; Wolmarans, L. Spectrophotometric absorbance of follicular fluid: A selection criterion. J. Assist. Reprod. Genet. 1992, 9, 539–544. [Google Scholar] [CrossRef]
- Morelli, C.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Ostuni, A. Metabolic changes in follicular fluids of patients treated with recombinant versus urinary human chorionic gonadotropin for triggering ovulation in assisted reproductive technologies: A metabolomics pilot study. Arch. Gynecol. Obstet. 2020, 302, 741–751. [Google Scholar] [CrossRef]
- Pocate-Cheriet, K.; Santulli, P.; Kateb, F.; Bourdon, M.; Maignien, C.; Batteux, F.; Chouzenoux, S.; Patrat, C.; Wolf, J.P.; Bertho, G. The follicular fluid metabolome differs according to the endometriosis phenotype. Reprod. Biomed. Online 2020, 41, 1023–1037. [Google Scholar] [CrossRef]
- Gérard, N.; Loiseau, S.; Duchamp, G.; Seguin, F. Analysis of the variations of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (1H NMR). Reprod. Camb. 2002, 124, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ogawa, H.; Fujioka, M.; Gerton, G.L. Guanidinoacetate methyltransferase in the mouse: Extensive expression in Sertoli cells of testis and in microvilli of caput epididymis. Biol. Reprod. 1994, 50, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewell, D.; Harris, R. Effects of creatine supplementation in the Thoroughbred horse. Equine Vet. J. 1995, 27, 239–242. [Google Scholar] [CrossRef]
- González-Fernández, L.; Sánchez-Calabuig, M.J.; Calle-Guisado, V.; García-Marín, L.J.; Bragado, M.J.; Fernández-Hernández, P.; Gutiérrez-Adán, A.; Macías-García, B. Stage-specific metabolomic changes in equine oviductal fluid: New insights into the equine fertilization environment. Theriogenology 2020, 143, 35–43. [Google Scholar] [CrossRef]
- Umehara, T.; Tsujita, N.; Goto, M.; Tonai, S.; Nakanishi, T.; Yamashita, Y.; Shimada, M. Methyl-beta cyclodextrin and creatine work synergistically under hypoxic conditions to improve the fertilization ability of boar ejaculated sperm. Anim. Sci. J. 2020, 91, e13493. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.M.; Friedman, D.L.; Grant, J.W.; Perryman, M.B.; Strauss, A.W. Creatine kinase isoenzymes are highly regulated during pregnancy in rat uterus and placenta. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E624–E635. [Google Scholar] [CrossRef] [PubMed]
- Satyaswaroop, P.; Mortel, R. Creatine kinase activity in human endometrium: Relative distribution in isolated glands and stroma. Am. J. Obstet. Gynecol. 1983, 146, 159–162. [Google Scholar] [CrossRef]
- Philip, M.; Snow, R.J.; Della Gatta, P.A.; Bellofiore, N.; Ellery, S.J. Creatine metabolism in the uterus: Potential implications for reproductive biology. Amino Acids 2020, 52, 1275–1283. [Google Scholar] [CrossRef]
- Subramani, E.; Jothiramajayam, M.; Dutta, M.; Chakravorty, D.; Joshi, M.; Srivastava, S.; Mukherjee, A.; Datta Ray, C.; Chakravarty, B.; Chaudhury, K. NMR-based metabonomics for understanding the influence of dormant female genital tuberculosis on metabolism of the human endometrium. Hum. Reprod. 2016, 31, 854–865. [Google Scholar] [CrossRef] [Green Version]
- Scambia, G.; Kaye, A.; Iacobelli, S. Creatine kinase BB in normal, hyperplastic and neoplastic endometrium. J. Steroid Biochem. 1984, 20, 797–798. [Google Scholar] [CrossRef]
- Borthwick, J.M.; Charnock-Jones, D.S.; Tom, B.D.; Hull, M.L.; Teirney, R.; Phillips, S.C.; Smith, S.K. Determination of the transcript profile of human endometrium. MHR: Basic Sci. Reprod. Med. 2003, 9, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhang, A.; Yu, F.; Gao, J.; Liu, Y.; Yu, C.; Zhou, H.; Xu, C. Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J. Proteome Res. 2015, 14, 1831–1842. [Google Scholar] [CrossRef]
- Choe, C.-u.; Nabuurs, C.; Stockebrand, M.C.; Neu, A.; Nunes, P.; Morellini, F.; Sauter, K.; Schillemeit, S.; Hermans-Borgmeyer, I.; Marescau, B. L-arginine: Glycine amidinotransferase deficiency protects from metabolic syndrome. Hum. Mol. Genet. 2013, 22, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandrì, M.G.; Strigini, F.; Cioni, G.; Battini, R. Increased creatine demand during pregnancy in Arginine: Glycine Amidino-Transferase deficiency: A case report. BMC Pregnancy Childbirth 2020, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Bick, J.; Ulbrich, S.E.; Bauersachs, S. Cell type-specific analysis of transcriptome changes in the porcine endometrium on Day 12 of pregnancy. BMC Genom. 2018, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Weisman, Y.; Golander, A.; Binderman, I.; Spirer, Z.; Kaye, A.; Sömjen, D. Stimulation of creatine kinase activity by calcium-regulating hormones in explants of human amnion, decidua, and placenta. J. Clin. Endocrinol. Metab. 1986, 63, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Franczak, A.; Wojciechowicz, B.; Kotwica, G. Transcriptomic analysis of the porcine endometrium during early pregnancy and the estrous cycle. Reprod. Biol. 2013, 13, 229–237. [Google Scholar] [CrossRef]
- Walker, J.B.; Gipson, W.T. Occurrence of transamidinase in decidua and its repression by dietary creatine. Biochim. Biophys. Acta (BBA) Spec. Sect. Enzymol. Subj. 1963, 67, 156–157. [Google Scholar] [CrossRef]
- Baharom, S.; De Matteo, R.; Ellery, S.; Della Gatta, P.; Bruce, C.R.; Kowalski, G.M.; Hale, N.; Dickinson, H.; Harding, R.; Walker, D. Does maternal-fetal transfer of creatine occur in pregnant sheep? Am. J. Physiol. Endocrinol. Metab. 2017, 313, E75–E83. [Google Scholar] [CrossRef]
- Emery, A.E.; Pascasio, F.M. The effects of pregnancy on the concentration of creatine kinase in serum, skeletal muscle, and myometrium. Am. J. Obstet. Gynecol. 1965, 91, 18–22. [Google Scholar] [CrossRef]
- Steingrimsdottir, T.; Ericsson, A.; Franck, A.; Waldenström, A.; Ulmsten, U.; Ronquist, G. Human uterine smooth muscle exhibits a very low phosphocreatine/ATP ratio as assessed by in vitro and in vivo measurements. Eur. J. Clin. Investig. 1997, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.M.; Seifter, S. Uptake of creatine by cultured cells. Arch. Biochem. Biophys. 1980, 203, 317–324. [Google Scholar] [CrossRef]
- Charpigny, G.; Leroy, M.-J.; Breuiller-Fouché, M.; Tanfin, Z.; Mhaouty-Kodja, S.; Robin, P.; Leiber, D.; Cohen-Tannoudji, J.; Cabrol, D.; Barberis, C. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol. Reprod. 2003, 68, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, M.; Fluellen, C.; Iyengar, C. Increased creatine kinase in the hormone-stimulated smooth muscle of the bovine uterus. Biochem. Biophys. Res. Commun. 1980, 94, 948–954. [Google Scholar] [CrossRef]
- Noyszewski, E.A.; Raman, J.; Trupin, S.R.; McFarlin, B.L.; Dawson, M.J. Phosphorus 31 nuclear magnetic resonance examination of female reproductive tissues. Am. J. Obstet. Gynecol. 1989, 161, 282–288. [Google Scholar] [CrossRef]
- Clark, J.F.; Khuchua, Z.; Kuznetsov, A.; Saks, V.; Ventura-Clapier, R. Compartmentation of creatine kinase isoenzymes in myometrium of gravid guinea-pig. J. Physiol. 1993, 466, 553–572. [Google Scholar]
- Dawson, M.J.; Wray, S. The effects of pregnancy and parturition on phosphorus metabolites in rat uterus studied by 31P nuclear magnetic resonance. J. Physiol. 1985, 368, 19–31. [Google Scholar] [CrossRef]
- Butte, N.F.; Wong, W.W.; Treuth, M.S.; Ellis, K.J.; O’Brian Smith, E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 2004, 79, 1078–1087. [Google Scholar] [CrossRef]
- Vaughan, O.; Fowden, A. Placental metabolism: Substrate requirements and the response to stress. Reprod. Domest. Anim. 2016, 51, 25–35. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Smith, R.; Maiti, K.; Aitken, R. Unexplained antepartum stillbirth: A consequence of placental aging? Placenta 2013, 34, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 2000, 54, S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Alvino, G.; Cardellicchio, M. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. 2009, 587, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Thomure, M. Regulation of creatine kinase isoenzymes in human placenta during early, mid-, and late gestation. J. Soc. Gynaecol. Investig. 1996, 3, 322–327. [Google Scholar] [CrossRef]
- Ellery, S.J.; Della Gatta, P.A.; Bruce, C.R.; Kowalski, G.M.; Davies-Tuck, M.; Mockler, J.C.; Murthi, P.; Walker, D.W.; Snow, R.J.; Dickinson, H. Creatine biosynthesis and transport by the term human placenta. Placenta 2017, 52, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingdom, J.C.P.; Kaufmann, P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 1997, 18, 613–621. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Camm, E.J. The Programming Power of the Placenta. Front. Physiol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Tissot van Patot, M.C.; Murray, A.J.; Beckey, V.; Cindrova-Davies, T.; Johns, J.; Zwerdlinger, L.; Jauniaux, E.; Burton, G.J.; Serkova, N.J. Human placental metabolic adaptation to chronic hypoxia, high altitude: Hypoxic preconditioning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R166–R172. [Google Scholar] [CrossRef] [Green Version]
- Krishna, U.; Bhalerao, S. Placental insufficiency and fetal growth restriction. J. Obstet. Gynecol. India 2011, 61, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, T.; Kärkkäinen, O.; Jokkala, J.; Litonius, K.; Heinonen, S.; Auriola, S.; Lehtonen, M.; Hanhineva, K.; Laivuori, H.; Kajantie, E.; et al. A Non-Targeted LC-MS Profiling Reveals Elevated Levels of Carnitine Precursors and Trimethylated Compounds in the Cord Plasma of Pre-Eclamptic Infants. Sci. Rep. 2018, 8, 14616. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.L.; Guan, X.-J.; Ingram, R.; Tilghman, S.M. Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc. Natl. Acad. Sci. USA 2003, 100, 4622–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.; Haig, D. Genomic imprinting in mammalian development: A parental tug-of-war. Trends Genet. 1991, 7, 45–49. [Google Scholar] [CrossRef]
- McMinn, J.; Wei, M.; Schupf, N.; Cusmai, J.; Johnson, E.; Smith, A.; Weksberg, R.; Thaker, H.; Tycko, B. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta 2006, 27, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Chard, T. Placental synthesis. Clin. Obstet. Gynaecol. 1986, 13, 447. [Google Scholar]
- Carter, A. Placental oxygen consumption. Part I: In vivo studies—A review. Placenta 2000, 21, S31–S37. [Google Scholar] [CrossRef]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental mitochondrial function and structure in gestational disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Holland, O.J.; Cuffe, J.S.M.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K.A.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
- Sferruzzi-Perri, A.N.; Higgins, J.S.; Vaughan, O.R.; Murray, A.J.; Fowden, A.L. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc. Natl. Acad. Sci. USA 2019, 116, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, H.; Walker, D. Managing a colony of spiny mice (Acomys cahirinus) for perinatal research. Aust. N. Z. Counc. Care Anim. Res. Train. (ANZCCART) News 2007, 20, 4–11. [Google Scholar]
- Bahado-Singh, R.O.; Akolekar, R.; Chelliah, A.; Mandal, R.; Dong, E.; Kruger, M.; Wishart, D.S.; Nicolaides, K. Metabolomic analysis for first-trimester trisomy 18 detection. Am. J. Obstet. Gynecol. 2013, 209, 65.e1–65.e9. [Google Scholar] [CrossRef] [PubMed]
- Marescau, B.; De Deyn, P.P.; Holvoet, J.; Possemiers, I.; Nagels, G.; Saxena, V.; Mahler, C. Guanidino compounds in serum and urine of cirrhotic patients. Metabolism 1995, 44, 584–588. [Google Scholar] [CrossRef]
- Class, S.; Class, S. Human Metabolome Database. Enzyme 2006, 1, 14. [Google Scholar]
- Dickinson, H.; Davies-Tuck, M.; Ellery, S.; Grieger, J.; Wallace, E.; Snow, R.; Walker, D.; Clifton, V. Maternal creatine in pregnancy: A retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1830–1838. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.; Barros, A.n.S.; Domingues, M.R.r.M.; Goodfellow, B.J.; Galhano, E.l.; Pita, C.; Almeida, M.d.C.; Carreira, I.M.; Gil, A.M. Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine. J. Proteome Res. 2015, 14, 1263–1274. [Google Scholar] [CrossRef]
- Heazell, A.E.; Bernatavicius, G.; Warrander, L.; Brown, M.C.; Dunn, W.B. A metabolomic approach identifies differences in maternal serum in third trimester pregnancies that end in poor perinatal outcome. Reprod. Sci. 2012, 19, 863–875. [Google Scholar] [CrossRef]
- De Guingand, D.L.; Ellery, S.J.; Davies-Tuck, M.L.; Dickinson, H. Creatine and pregnancy outcomes, a prospective cohort study in low-risk pregnant women: Study protocol. BMJ Open 2019, 9, e026756. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.; Hanson, R.; Yancey, P. Developmental changes in organic osmolytes in prenatal and postnatal rat tissues. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2000, 125, 45–56. [Google Scholar] [CrossRef]
- Kreis, R.; Hofmann, L.; Kuhlmann, B.; Boesch, C.; Bossi, E.; Hüppi, P.S. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 2002, 48, 949–958. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Villard, A.-M.; Speer, O.; Wallimann, T.; Bachmann, C. Creatine synthesis and transport during rat embryogenesis: Spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev. Biol. 2005, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braissant, O.; Henry, H.; Loup, M.; Eilers, B.; Bachmann, C. Endogenous synthesis and transport of creatine in the rat brain: An in situ hybridization study. Mol. Brain Res. 2001, 86, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Bachmann, C.; Henry, H. Expression and function of AGAT, GAMT and CT1 in the mammalian brain. In Creatine and Creatine Kinase in Health and Disease; Springer: Dordrecht, The Netherlands, 2007; pp. 67–81. [Google Scholar]
- Evangelou, I.E.; Du Plessis, A.J.; Vezina, G.; Noeske, R.; Limperopoulos, C. Elucidating metabolic maturation in the healthy fetal brain using 1H-MR spectroscopy. Am. J. Neuroradiol. 2015, 37, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, C.R.; Van Der Zee, C.E.; In ’t Zandt, H.J.; Oerlemans, F.; Verheij, M.; Streijger, F.; Fransen, J.; Heerschap, A.; Cools, A.R.; Wieringa, B. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur. J. Neurosci. 2002, 15, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Zanolla, E.; Furter-Graves, E.M.; Eppenberger, H.M.; Wallimann, T. Creatine Kinase Isoenzymes in Chicken Cerebellum: Specific Localization of Brain-type Creatine Kinase in Bergmann Glial Cells and Muscle-type Creatine Kinase in Purkinje Neurons. Eur. J. Neurosci. 1994, 6, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Carducci, C.; Carducci, C.; Santagata, S.; Adriano, E.; Artiola, C.; Thellung, S.; Gatta, E.; Robello, M.; Florio, T.; Antonozzi, I. In vitro study of uptake and synthesis of creatine and its precursors by cerebellar granule cells and astrocytes suggests some hypotheses on the physiopathology of the inherited disorders of creatine metabolism. BMC Neurosci. 2012, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyoo, I.K.; Kong, S.W.; Sung, S.M.; Hirashima, F.; Parow, A.; Hennen, J.; Cohen, B.M.; Renshaw, P.F. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003, 123, 87–100. [Google Scholar] [CrossRef]
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, M.; Fukaya, M.; Terasaki, T.; Ohtsuki, S.; Watanabe, M. Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron–glial relationship for brain energy homeostasis. Eur. J. Neurosci. 2004, 20, 144–160. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1646, discussion 1647. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Rosenberg, P.A. Pathophysiology of glia in perinatal white matter injury. Glia 2014, 62, 1790–1815. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, K.A.; Chapey, K.S.; Nanescu, S.E.; Huang, J.K. Creatine enhances mitochondrial-mediated oligodendrocyte survival after demyelinating injury. J. Neurosci. 2017, 37, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braissant, O.; Béard, E.; Torrent, C.; Henry, H. Dissociation of AGAT, GAMT and SLC6A8 in CNS: Relevance to creatine deficiency syndromes. Neurobiol. Dis. 2010, 37, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Sartini, S.; Lattanzi, D.; Ambrogini, P.; Di Palma, M.; Galati, C.; Savelli, D.; Polidori, E.; Calcabrini, C.; Rocchi, M.; Sestili, P. Maternal creatine supplementation affects the morpho-functional development of hippocampal neurons in rat offspring. Neuroscience 2016, 312, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ellery, S.J.; Dickinson, H.; McKenzie, M.; Walker, D.W. Dietary interventions designed to protect the perinatal brain from hypoxic-ischemic encephalopathy–creatine prophylaxis and the need for multi-organ protection. Neurochem. Int. 2016, 95, 15–23. [Google Scholar] [CrossRef]
- Beal, M.F. Neuroprotective effects of creatine. Amino Acids 2011, 40, 1305–1313. [Google Scholar] [CrossRef]
- Genius, J.; Geiger, J.; Bender, A.; Möller, H.-J.; Klopstock, T.; Rujescu, D. Creatine protects against excitoxicity in an in vitro model of neurodegeneration. PLoS ONE 2012, 7, e30554. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, D.; Tsuji, M.; Wallimann, T.; Hemmer, W. Functional maturation of creatine kinase in rat brain. Dev. Neurosci. 1993, 15, 261–270. [Google Scholar] [CrossRef]
- Prass, K.; Royl, G.; Lindauer, U.; Freyer, D.; Megow, D.; Dirnagl, U.; Stöckler-Ipsiroglu, G.; Wallimann, T.; Priller, J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J. Cereb. Blood Flow Metab. 2006, 27, 452–459. [Google Scholar] [CrossRef]
- Ireland, Z.; Castillo-Melendez, M.; Dickinson, H.; Snow, R.; Walker, D. A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience 2011, 194, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Middelanis, J.; Vaihinger, H.-M.; Mies, G.; Wilken, B.; Jensen, A. Creatine protects the immature brain from hypoxic-ischemic injury. J. Soc. Gynecol. Investig. 2004, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wilken, B.; Ramirez, J.; Probst, I.; Richter, D.; Hanefeld, F. Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr. Res. 1998, 43, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzman, D.; Togliatti, A.; Khait, I.; Jensen, F. Creatine Increases Survival and Suppresses Seizures in the Hypoxic Immature Rat. Pediatr. Res. 1998, 44, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.; Khait, I.; Mulkern, R.; Allred, E.; Rand, T.; Jensen, F.; Kraft, R. In vivo development of brain phosphocreatine in normal and creatine-treated rabbit pups. J. Neurochem. 1999, 73, 2477–2484. [Google Scholar] [CrossRef] [Green Version]
- Adcock, K.H.; Nedelcu, J.; Loenneker, T.; Martin, E.; Wallimann, T.; Wagner, B.P. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev. Neurosci. 2002, 24, 382–388. [Google Scholar] [CrossRef]
- Ireland, Z.; Dickinson, H.; Snow, R.; Walker, D.W. Maternal creatine: Does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am. J. Obstet. Gynecol. 2008, 198, 431–436. [Google Scholar] [CrossRef]
- Cannata, D.J.; Ireland, Z.; Dickinson, H.; Snow, R.J.; Russell, A.P.; West, J.M.; Walker, D.W. Maternal Creatine Supplementation From Mid-Pregnancy Protects the Diaphragm of the Newborn Spiny Mouse From Intrapartum Hypoxia-Induced Damage. Pediatr. Res. 2010, 68, 393–398. [Google Scholar] [CrossRef] [Green Version]
- LaRosa, D.A.; Ellery, S.J.; Parkington, H.C.; Snow, R.J.; Walker, D.W.; Dickinson, H. Maternal Creatine Supplementation during Pregnancy Prevents Long-Term Changes in Diaphragm Muscle Structure and Function after Birth Asphyxia. PLoS ONE 2016, 11, e0149840. [Google Scholar] [CrossRef] [Green Version]
- LaRosa, D.A.; Ellery, S.J.; Snow, R.J.; Walker, D.W.; Dickinson, H. Maternal creatine supplementation during pregnancy prevents acute and long-term deficits in skeletal muscle after birth asphyxia: A study of structure and function of hind limb muscle in the spiny mouse. Pediatr. Res. 2016, 80, 852–860. [Google Scholar] [CrossRef]
- Ellery, S.J.; Ireland, Z.; Kett, M.M.; Snow, R.; Walker, D.W.; Dickinson, H. Creatine pretreatment prevents birth asphyxia-induced injury of the newborn spiny mouse kidney. Pediatr. Res. 2013, 73, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ellery, S.J.; LaRosa, D.A.; Cullen-McEwen, L.A.; Brown, R.D.; Snow, R.J.; Walker, D.W.; Kett, M.M.; Dickinson, H. Renal dysfunction in early adulthood following birth asphyxia in male spiny mice, and its amelioration by maternal creatine supplementation during pregnancy. Pediatr. Res. 2017, 81, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Hankins, G.D.; Koen, S.; Gei, A.F.; Lopez, S.M.; Van Hook, J.W.; Anderson, G.D. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet. Gynecol. 2002, 99, 688–691. [Google Scholar] [CrossRef]
- Steinbach, R.J.; Ellery, S.J.; Snow, R.J.; Walker, D.W.; Kempton, B.; Brigande, J.V.; Renner, L.; Neuringer, M.; Kroenke, C.D.; Schelonka, R.L. A Non-Human Primate Model of Hypoxic Ischemic Encephalopathy to Evaluate Novel Translational Therapeutics. In Proceedings of Reproductive Sciences; Springer: Heidelberg, Germany, 2020; p. 151A. [Google Scholar]
- Braissant, O.; Henry, H.; Béard, E.; Uldry, J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 2011, 40, 1315–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüml, S.; Wisnowski, J.L.; Nelson Jr, M.D.; Paquette, L.; Panigrahy, A. Metabolic maturation of white matter is altered in preterm infants. PLoS ONE 2014, 9, e85829. [Google Scholar] [CrossRef]
- Blüml, S.; Wisnowski, J.L.; Nelson Jr, M.D.; Paquette, L.; Gilles, F.H.; Kinney, H.C.; Panigrahy, A. Metabolic maturation of the human brain from birth through adolescence: Insights from in vivo magnetic resonance spectroscopy. Cereb. Cortex 2012, 23, 2944–2955. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.S.; Rosenberg, E.H.; Verhoeven, N.M.; Jakobs, C.; Salomons, G.S. Are cerebral creatine deficiency syndromes on the radar screen? Future Neurol. 2006, 1, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Battini, R.; Alessandri, M.; Leuzzi, V.; Moro, F.; Tosetti, M.; Bianchi, M.; Cioni, G. Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: Early treatment can prevent phenotypic expression of the disease. J. Paediatr. 2006, 148, 828–830. [Google Scholar] [CrossRef]
- Rostami, P.; Hosseinpour, S.; Ashrafi, M.R.; Alizadeh, H.; Garshasbi, M.; Tavasoli, A.R. Primary creatine deficiency syndrome as a potential missed diagnosis in children with psychomotor delay and seizure: Case presentation with two novel variants and literature review. Acta Neurol. Belg. 2020, 120, 511–516. [Google Scholar] [CrossRef]
- Edison, E.E.; Brosnan, M.E.; Aziz, K.; Brosnan, J.T. Creatine and guanidinoacetate content of human milk and infant formulas: Implications for creatine deficiency syndromes and amino acid metabolism. Br. J. Nutr. 2013, 110, 1075–1078. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, H.; Ireland, Z.J.; LaRosa, D.A.; O’Connell, B.A.; Ellery, S.; Snow, R.; Walker, D.W. Maternal dietary creatine supplementation does not alter the capacity for creatine synthesis in the newborn spiny mouse. Reprod. Sci. 2013, 20, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.J.; Foster, T.; Rowe, K.; Robertson, O.; Robson, B.; Pierse, N. Gestational age, health, and educational outcomes in adolescents. Pediatrics 2018, 142, e20181016. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lu, Y.-C.; Jacobs, M.; Pradhan, S.; Kapse, K.; Zhao, L.; Niforatos-Andescavage, N.; Vezina, G.; du Plessis, A.J.; Limperopoulos, C. Association of Prenatal Maternal Psychological Distress With Fetal Brain Growth, Metabolism, and Cortical Maturation. JAMA Netw. Open 2020, 3, e1919940. [Google Scholar] [CrossRef] [PubMed]
- Koob, M.; Viola, A.; Le Fur, Y.; Viout, P.; Ratiney, H.; Confort-Gouny, S.; Cozzone, P.J.; Girard, N. Creatine, glutamine plus glutamate, and macromolecules are decreased in the central white matter of premature neonates around term. PLoS ONE 2016, 11, e0160990. [Google Scholar] [CrossRef]
- Lage, S.; Andrade, F.; Prieto, J.A.; Asla, I.; Rodríguez, A.; Ruiz, N.; Echeverría, J.; Luz Couce, M.; Sanjurjo, P.; Aldámiz-Echevarría, L. Arginine-guanidinoacetate-creatine pathway in preterm newborns: Creatine biosynthesis in newborns. J. Pediatr. Endocrinol. Metab. 2013, 26, 53–60. [Google Scholar] [CrossRef]
- Berry, M.J.; Schlegel, M.; Kowalski, G.M.; Bruce, C.R.; Callahan, D.L.; Davies-Tuck, M.L.; Dickinson, H.; Goodson, A.; Slocombe, A.; Snow, R.J. UNICORN Babies: Understanding Circulating and Cerebral Creatine Levels of the Preterm Infant. An Observational Study Protocol. Front. Physiol. 2019, 10, 142. [Google Scholar] [CrossRef]
- De Guingand, D.L.; Palmer, K.R.; Bilardi, J.E.; Ellery, S.J. Acceptability of dietary or nutritional supplementation in pregnancy (ADONS)–Exploring the consumer’s perspective on introducing creatine monohydrate as a pregnancy supplement. Midwifery 2020, 82, 102599. [Google Scholar] [CrossRef]
- De Guingand, D.L.; Palmer, K.R.; Snow, R.J.; Davies-Tuck, M.L.; Ellery, S.J. Risk of adverse outcomes in females taking oral creatine monohydrate: A systematic review and meta-analysis. Nutrients 2020, 12, 1780. [Google Scholar] [CrossRef]
- Bohnhorst, B.; Geuting, T.; Peter, C.S.; Dördelmann, M.; Wilken, B.; Poets, C.F. Randomized, controlled trial of oral creatine supplementation (not effective) for apnea of prematurity. Pediatrics 2004, 113, e303–e307. [Google Scholar] [CrossRef] [Green Version]


| Tissue | Species | Creatine and Phosphocreatine Content | Creatine Kinases | Creatine Synthesis and Transport |
|---|---|---|---|---|
| Oocytes | Mouse | Creatine and phosphocreatine present (~4 to 5 mmol.kg−1 dry mass) [16]. | CKBB gene, protein and activity reported [16,17]. Activity increased with oocyte maturation and fertilization [18]. CKMM detected. Expression levels increased with hCG stimulation [9]. | |
| Bovine | uMt-CK and CKBB gene and protein expression reported [18]. Use of CK inhibitors elevated intra-oocyte ADP:ATP ratio [19]. | |||
| Human | Creatine and phosphocreatine present (~4 to 5 mmol.kg−1 dry mass) [16]. | |||
| Ovaries | Rat | High SLC6A8 gene expression reported [21]. | ||
| Ovarian stromal cells | Human | Detectable levels of the GATM gene and AGAT protein, but GAMT undetected [22]. | ||
| Cumulus cells or cumulus–oocyte complexes (COCs) | Human | CKBB gene expression detected and elevated in women with good quality embryos undergoing ART [23]. | ||
| Bovine | Creatine and GAA detected in media bathing cells, with an increase in creatine (~450-fold) and GAA (~2-fold) reported during in vitro maturation [24]. | |||
| Follicular fluid | Human | Creatine detected and lower in women with endometrioma [30]. | ||
| Mouse | Creatine detected and increases around ovulation [9]. | |||
| Equine | Creatine detected. Remains unchanged with follicular development [31]. | |||
| Granulosa cells | Rat | Increase in GATM and GAMT expression with equine CG stimulation [9]. | ||
| Oviduct | Human | GATM and GAMT and SLC6A8 detected [21,22] | ||
| Rat | GATM and GAMT and SLC6A8 detected [21,22] | |||
| Mouse | GAMT gene and protein not expressed [32] | |||
| Oviductal fluid | Equine | High creatine concentration (3–4 mM) that did not change pre- to post-ovulation [33,34]. | ||
| Mouse | Creatine levels detected and increased with hCG stimulation [9]. | GATM and GAMT detected. No change in expression with hCG stimulation [9]. | ||
| Non-pregnant Endometrium | Human | Up-regulation of CKBB expression and enzyme activity in the secretory phase of the menstrual cycle [37,39,40,41]. | Increased SLC6A8 expression during the secretory phase of the menstrual cycle [37]. | |
| Pregnant endometrium | Rat | uMt-CK and CKBB proteins expressed in the decidua parietalis and basalis [36]. | AGAT activity high in the decidua. No GAMT enzyme activity present [48]. | |
| Sheep | GAA produced at a higher level than non-pregnant animals [49]. | |||
| Human | Creatine kinase activity present in term decidual explants [46]. | |||
| Non-pregnant myometrium | Human | Phosphocreatine detected at a low level compared pregnant myometrium [51]. | Creatine kinase activity detected [50]. | |
| Pregnant myometrium | Human | Phosphocreatine detected with higher levels at term compared to non-pregnant tissue [55]. | CKBB gene expression detected. Levels were three-fold higher at term compared with earlier in gestation [53]. |
| Study | Condition | Gestation | Creatine and Phosphocreatine Content | Creatine Kinases | Creatine Synthesis and Transport |
|---|---|---|---|---|---|
| Thomure et al. [64] | Healthy | First, second and third trimester | uMt-CK and CKBB gene expression detected. Expression was low in the first and second trimester before a peak at term. CKBB protein expression consistent throughout gestation. uMt-CK expression rose through to mid-gestation before declining just before term. | ||
| Ellery et al. [13] | Healthy | First trimester (10–13 weeks’ gestation) | GATM, GAMT and SLC6A8 detected. | ||
| Ellery et al. [13,65] | Healthy | Third trimester | AGAT, GAMT and SLC6A8 gene and protein detected. GATM expression and GAA tissue content decreased with advancing gestational age and birth weight. | ||
| Tissot et al. [69] | High altitude | Term | Increased phosphocreatine levels detected. | ||
| Ellery et al. [13] | FGR | Third trimester | 43% higher total creatine content compared to gestation-matched controls. | 2-fold increase in SLC6A8 expression. | |
| Ellery et al. [14] | PE | Third trimester | 38% higher total creatine content compared to gestation-matched controls. | Increased CKBB mRNA expression. | Increased GATM, GAMT, SLC6A8 mRNA expression. |
| Jääskeläinen et al. [71] | PE | Term | Increase in creatine concentration in venous cord plasma at delivery. | ||
| McMinn et al. [74] | FGR | Term | Down-regulation in GATM. |
| Species | Developmental Timing | Treatment | Main Outcomes |
|---|---|---|---|
| Guinea pigs and Rats [113] | Fetal guinea pigs (0.9 gestation) Or neonatal rats (P7) | 2 h creatine treatment to hippocampal slices in vitro or injection of 3g/kg creatine before and after hypoxic-ischemic insult. | Creatine improved recovery of brain protein synthesis, reduced infarction and neuronal cell injury. |
| Mice [114] | Neonatal (P0–5) and juvenile (P6–13) | Maternal dietary creatine supplementation (2 g/kg/day) or incubation of brain slices (200 μM) creatine. | Creatine preserved ATP turnover and reduced neuronal injury. |
| Rat [115] | Neonatal or juvenile (P10–15) | Subcutaneous creatine (3 mg/g of body weight) for 3 days before hypoxic insult. | Low phosphocreatine/creatine ratio led to higher susceptibility of seizures. Creatine improved survival and prevented seizure activity. |
| Rabbit [116] | 5 to 30 day-old pups | Subcutaneous creatine (3 mg/g of body weight) for 3 days before hypoxic insult. | Creatine increased brain PCr/NTP ratio and prevented hypoxic seizures. |
| Rat [117] | Neonatal (P6) | Subcutaneous creatine (3 g/kg body weight/day) for 3 days before hypoxic insult. | Creatine prevented brain oedema associated with severe hypoxia-ischemia. |
| Spiny Mouse [118] | Fetal (term) and juvenile (P15) | Maternal dietary creatine supplementation (5% w/w from mid-gestation). | Creatine increased pup survival and improved postnatal growth. |
| Spiny Mouse [118] | Neonatal (P1) | Maternal dietary creatine supplementation (5% w/w from mid-gestation). | Creatine reduced perinatal mortality and pro-apoptotic protein BAX, cytoplasmic cytochrome c, and caspase-3 in the fetal brain. |
| Spiny Mouse [119,120,121,122] | Neonatal (P1) or juvenile (P35) | Maternal dietary creatine supplementation (5% w/w from mid-gestation). | Creatine prevented structural and functional damage to the diaphragm [119,120], skeletal muscle [121], and kidney [122]. |
| Spiny Mouse [123] | Adult (P90) | Maternal dietary creatine supplementation (5% w/w from mid-gestation). | Creatine decreased the risk of male offspring developing chronic kidney disease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020490
Muccini AM, Tran NT, de Guingand DL, Philip M, Della Gatta PA, Galinsky R, Sherman LS, Kelleher MA, Palmer KR, Berry MJ, et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients. 2021; 13(2):490. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020490
Chicago/Turabian StyleMuccini, Anna Maria, Nhi T. Tran, Deborah L. de Guingand, Mamatha Philip, Paul A. Della Gatta, Robert Galinsky, Larry S. Sherman, Meredith A. Kelleher, Kirsten R. Palmer, Mary J. Berry, and et al. 2021. "Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health" Nutrients 13, no. 2: 490. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020490