Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients
Abstract
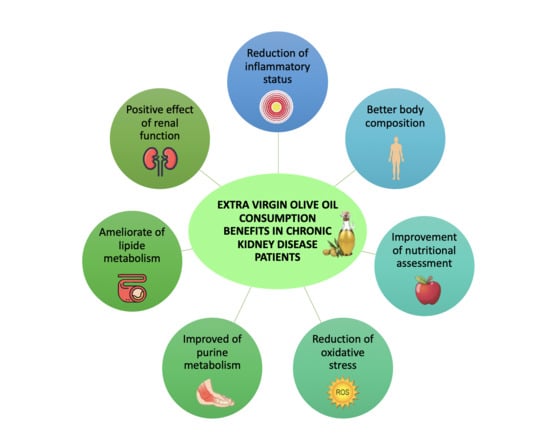
:1. Introduction
2. Material and Methods
2.1. Samples of EVOOs
2.2. Patients and Methods
2.3. Questionnaires
2.4. Body Composition Assessment
2.5. Laboratory Parameters
2.6. Statistical Analysis
3. Results
3.1. HPLC-DAD-MS: EVOOs Characterization
3.2. Study in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Ramos, C.I.; Teixeira, R.R.; Muniz, G.A.S.; Claudino, G.; Cuppari, L. Diet in Chronic Kidney Disease: An integrated approach to nutritional therapy. Rev. Assoc. Med. Bras. 2020, 66, s59–s67. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rysz, J.; Franczyk, B.; Cialkowska-Rysz, A.; Gluba-Brzozka, A. The Effect of Diet on the Survival of Patients with Chronic Kidney Disease. Nutrients 2017, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.J.; Campbell, K.L.; Strippoli, G.F. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Lew, Q.J.; Jafar, T.H.; Koh, H.W.; Jin, A.; Chow, K.Y.; Yuan, J.M.; Koh, W.P. Red Meat Intake and Risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef]
- Andreoli, A.; Lauro, S.; Di Daniele, N.; Sorge, R.; Celi, M.; Volpe, S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. Eur. J. Clin. Nutr. 2008, 62, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, S.; Ieri, F.; Romani, A. Minor polar compounds in extra virgin olive oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu spectrophotometric method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37, S66–S70. [Google Scholar] [CrossRef]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, M.; Probst-Hensch, N.; Kriemler, S.; Meier, F.; Autenrieth, C.; Martin, B.W. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev. Med. Rep. 2016, 3, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, R.L.; Sharbaugh, C.O.; Stapell, C.A. Reliability and validity of the 24-hour recall. J. Am. Diet. Assoc. 1981, 79, 542–547. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Reynaldo Martorell, H.M. Anthropometric Standardization Reference Manual; Human Kinetics: Champaign, IL, USA, 1988. [Google Scholar]
- Bellizzi, V.; Scalfi, L.; Terracciano, V.; De Nicola, L.; Minutolo, R.; Marra, M.; Guida, B.; Cianciaruso, B.; Conte, G.; Di Iorio, B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Gaman, M.A.; Epingeac, M.E.; Diaconu, C.C.; Gaman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes 2020, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar]
- Lewis, N.A.; Newell, J.; Burden, R.; Howatson, G.; Pedlar, C.R. Critical Difference and Biological Variation in Biomarkers of Oxidative Stress and Nutritional Status in Athletes. PLoS ONE 2016, 11, e0149927. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper respiratory tract health” (ID 3468), “can help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 5 August 2020).
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Paiva-Martins, F.; Corona, G.; Debnam, E.S.; Jose Oruna-Concha, M.; Vauzour, D.; Gordon, M.H.; Spencer, J.P. Absorption and metabolism of olive oil secoiridoids in the small intestine. Br. J. Nutr. 2011, 105, 1607–1618. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F.; Bernardini, E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Zafra Gomez, A.; Luzón-Toro, B.; Capel-Cuevas, S.; Morales, J. Stability of Hydroxytyrosol in Aqueous Solutions at Different Concentration, Temperature and with Different Ionic Content: A Study Using UPLC-MS. Food Nutr. Sci. 2011, 2, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Lapucci, C.; Cantini, C.; Ieri, F.; Mulinacci, N.; Visioli, F. Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra virgin olive oil. J. Agric. Food Chem. 2007, 55, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santiago, M.; Fonolla, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.P.; Huang, X.T.; Sun, Y.; Mao, J.X.; Wang, S.; Wang, C.D.; Bao, Z.M. Molecular genetic analysis of heterosis in interspecific hybrids of Argopecten purpuratus x A. irradians irradians. Genet. Mol. Res. 2015, 14, 10692–10704. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Montes-Delgado, R.; Guerrero Riscos, M.A.; Garcia-Luna, P.P.; Martin Herrera, C.; Pereira Cunill, J.L.; Garrido Vazquez, M.; Lopez Munoz, I.; Suarez Garcia, M.J.; Martin-Espejo, J.L.; Soler Junco, M.L.; et al. [Treatment with low-protein diet and caloric supplements in patients with chronic kidney failure in predialysis. Comparative study]. Rev. Clin. Esp. 1998, 198, 580–586. [Google Scholar]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Wei-Jie, Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2016, 35, 117–124. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens 2004, 13, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Fabrini, R.; Dessi, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Tsai, C.W.; Lin, S.Y.; Kuo, C.C.; Huang, C.C. Serum Uric Acid and Progression of Kidney Disease: A Longitudinal Analysis and Mini-Review. PLoS ONE 2017, 12, e0170393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goicoechea, M.; Garcia de Vinuesa, S.; Verdalles, U.; Verde, E.; Macias, N.; Santos, A.; Perez de Jose, A.; Cedeno, S.; Linares, T.; Luno, J. Allopurinol and progression of CKD and cardiovascular events: Long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 2015, 65, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Harris, K.P. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis. 1996, 27, 765–775. [Google Scholar] [CrossRef]
- Coresh, J.; Heerspink, H.J.L.; Sang, Y.; Matsushita, K.; Arnlov, J.; Astor, B.C.; Black, C.; Brunskill, N.J.; Carrero, J.J.; Feldman, H.I.; et al. Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019, 7, 115–127. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef]
- Zubovic, S.V.; Kristic, S.; Prevljak, S.; Pasic, I.S. Chronic Kidney Disease and Lipid Disorders. Med. Arch. 2016, 70, 191–192. [Google Scholar] [CrossRef] [Green Version]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; Manca di Villahermosa, S.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tsartsou, E.; Proutsos, N.; Castanas, E.; Kampa, M. Network Meta-Analysis of Metabolic Effects of Olive-Oil in Humans Shows the Importance of Olive Oil Consumption With Moderate Polyphenol Levels as Part of the Mediterranean Diet. Front. Nutr. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Obermayr, R.P.; Temml, C.; Gutjahr, G.; Knechtelsdorfer, M.; Oberbauer, R.; Klauser-Braun, R. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008, 19, 2407–2413. [Google Scholar] [CrossRef] [Green Version]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Spiga, R.; Marini, M.A.; Mancuso, E.; Di Fatta, C.; Fuoco, A.; Perticone, F.; Andreozzi, F.; Mannino, G.C.; Sesti, G. Uric Acid Is Associated With Inflammatory Biomarkers and Induces Inflammation Via Activating the NF-kappaB Signaling Pathway in HepG2 Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Reyes, C.P.; Manjarrez-Reyna, A.N.; Mendez-Garcia, L.A.; Aguayo-Guerrero, J.A.; Aguirre-Sierra, B.; Villalobos-Molina, R.; Lopez-Vidal, Y.; Bobadilla, K.; Escobedo, G. Uric Acid Has Direct Proinflammatory Effects on Human Macrophages by Increasing Proinflammatory Mediators and Bacterial Phagocytosis Probably via URAT1. Biomolecules 2020, 10, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Liu, X.; Lv, Q.; Ren, H.; Gao, L.; Zhao, P.; Yang, X.; Yang, G.; Xu, D.; Wang, G.; Yang, W.; et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ 2020, 8, e8664. [Google Scholar] [CrossRef]
- Galvao Candido, F.; Xavier Valente, F.; da Silva, L.E.; Goncalves Leao Coelho, O.; Gouveia Peluzio, M.D.C.; Goncalves Alfenas, R.C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2018, 57, 2445–2455. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Barnett, N.W.; Sinclair, A.J.; Keast, R.S. Influence of heat on biological activity and concentration of oleocanthal--a natural anti-inflammatory agent in virgin olive oil. J. Agric. Food Chem. 2009, 57, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, P.; Lopez-Miranda, J.; Blanco-Colio, L.; Bellido, C.; Jimenez, Y.; Moreno, J.A.; Delgado-Lista, J.; Egido, J.; Perez-Jimenez, F. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor kappaB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis 2007, 194, e141–e146. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Perez Banasco, V.; Gil-Cunquero, J.M.; Borrego, F.J.; Grasso, M.; Segura, P.; Warletta, F.; Lozano, J.L.; Costa, L.A.; Torres, J.; Gaforio, J.J.; et al. [Preliminary study on efficacy and tolerance of a “coupage” of olive oil in patients with chronic kidney disease. Nutritonal evaluation]. Nefrologia 2007, 27, 472–481. [Google Scholar] [PubMed]
- Villarrubia, V.; Vidal, S.; Borrego-Utiel, F.; Gil-Cunquero, J.; Pérez-Bañasco, V. A master formulation of organic extra virgin olive oils increases HDL cholesterol and albumin serum levels in kidney patients, and ameliorates atopic dermatitis in children and adults. Proc. Nutr. Soc. 2010. [Google Scholar] [CrossRef] [Green Version]
- Kaysen, G.A.; Dubin, J.A.; Muller, H.G.; Rosales, L.; Levin, N.W.; Mitch, W.E.; Niddk, H.S.G. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 2004, 65, 1408–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitozzi, V.; Jacomelli, M.; Zaid, M.; Luceri, C.; Bigagli, E.; Lodovici, M.; Ghelardini, C.; Vivoli, E.; Norcini, M.; Gianfriddo, M.; et al. Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br. J. Nutr 2010, 103, 1674–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Caruso, F.; Kwok, L.; Lee, G.; Caruso, A.; Gionfra, F.; Candelotti, E.; Belli, S.L.; Molasky, N.; Raley-Susman, K.M.; et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE 2017, 12, e0189341. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef]
- Talluri, A.; Liedtke, R.; Mohamed, E.I.; Maiolo, C.; Martinoli, R.; De Lorenzo, A. The application of body cell mass index for studying muscle mass changes in health and disease conditions. Acta Diabetol. 2003, 40, S286–S289. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Rovella, V.; Cusumano, A.; Di Daniele, N.; Casasco, M. Beneficial effects of physical activity on uremic sarcopenia. Med. Dello Sport 2018. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Rondanelli, M.; Talluri, J.; Peroni, G.; Donelli, C.; Guerriero, F.; Ferrini, K.; Riggi, E.; Sauta, E.; Perna, S.; Guido, D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin. Nutr. 2018, 37, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, R.; Mohamed, E.I.; Maiolo, C.; Cianci, R.; Denoth, F.; Salvadori, S.; Iacopino, L. Total body water estimation using bioelectrical impedance: A meta-analysis of the data available in the literature. Acta Diabetol. 2003, 40, S203–S206. [Google Scholar] [CrossRef] [PubMed]



| Compound | SYN | LUX |
|---|---|---|
| Hydroxytyrosol (mg/L *) | 1.78 ± 0.05 | 0.5 ± 0.02 |
| Tyrosol (mg/L *) | 1.71 ± 0.05 | 0.5 ± 0.03 |
| Elenolic acid derivatives (mg/L *) | 198.76 ± 5.96 | 32.26 ± 0.97 |
| Elenolic acid (mg/L *) | 28.15 ± 0.84 | 129.1 ± 3.87 |
| Oleacin (mg/L *) | 121.98 ± 3.66 | 77.54 ± 2.33 |
| Oleocanthal (mg/L *) | 46.02 ± 1.38 | 41.23 ± 1.24 |
| Oleuropein aglycone (mg/L *) | 142.65 ± 4.28 | 23.95 ± 0.72 |
| Secoiridoids derivatives (mg/L *) | 34.29 ± 1.03 | 87.47 ± 2.62 |
| Lignans (mg/L*) | 131.02 ± 3.93 | 92.44 ± 2.77 |
| Total MCP (mg/L*) | 706.36 ± 21.19 | 485.01 ± 14.55 |
| SYN | LUX | p (ANOVA Test) | |
|---|---|---|---|
| N | 20 | 20 | |
| Gender (male/female) | 13/7 | 12/8 | ns |
| Age (years) | 70.6 ± 12.4 | 68.5 ± 11.4 | ns |
| BMI (kg/m2) | 28.9 ± 3.7 | 27.5 ± 5.9 | ns |
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | |
| Creatinine (mg/dL) | 2.1 ± 0.8 | 2.0 ± 0.9 | ns # | 2.1 ± 1.0 | ns # | 2.2 ± 1.2 | 2.1 ± 1.2 | ns # | 2.2 ± 1.2 | ns # |
| e-GFR (mL/min/1.73 m2) | 34.5 ± 16.8 | 37.7 ± 18.7 | 0.0235 # | 38.2 ± 19.5 | ns # | 37.7 ± 20.0 | 40.2 ± 21.0 | ns # | 37.5 ± 20.0 | ns # |
| Albuminuria (mg/dL) | 31.8 ± 45.2 | 16.4 ± 24.1 | 0.0413 # | 28.8 ± 45.1 | ns # | 30.8 ± 59.6 | 18.8 ± 41.13 | ns # | 22.7 ± 55.2 | ns # |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.3 ± 0.3 | 0.0039 # | 4.3 ± 0.3 | ns # | 4.2 ± 0.3 | 4.4 ± 0.4 | 0.0442 # | 4.2 ± 0.3 | 0.0290 # |
| Azotaemia (mg/dL) | 69.6 ± 23.1 | 59.2 ± 17.3 | 0.0346 # | 61.3 ± 17.6 | ns # | 65.4 ± 18.8 | 60.3 ± 22.0 | ns # | 63.7 ± 21.9 | ns # |
| Sodium (mEq/L) | 139.7 ± 3.4 | 139.5 ± 2.5 | ns # | 139.6 ± 2.6 | ns # | 141.6 ± 1.9 | 140.1 ± 2.5 | ns # | 141.2 ± 1.8 | ns # |
| Potassium (mEq/L) | 4.4 ± 0.6 | 4.4 ± 0.6 | ns # | 4.3 ± 0.5 | ns # | 4.5 ± 0.4 | 4.4 ± 0.4 | ns # | 4.5 ± 0.5 | ns # |
| Calcium (mg/dL) | 9.8 ± 0.5 | 9.6 ± 0.4 | ns # | 9.6 ± 0.4 | ns # | 9.8 ± 0.4 | 9.6 ± 0.5 | ns # | 9.7 ± 0.4 | ns # |
| Phosphorus (mg/dL) | 3.4 ± 0.6 | 3.3 ± 0.6 | ns # | 3.3 ± 0.6 | ns # | 3.6 ± 0.6 | 3.5 ± 0.6 | ns # | 3.7 ± 0.7 | ns # |
| TC (mg/dL) | 175.1 ± 44.9 | 169.5 ± 37.5 | ns # | 147.7 ± 47.2 | ns # | 178.9 ± 56.1 | 181.0 ± 47.7 | ns # | 180.9 ± 52.6 | ns # |
| HDL-C (mg/dL) | 41.8 ± 13.3 | 41.3 ± 12.7 | ns # | 42.4 ± 11.7 | ns # | 44.5 ± 12.3 | 47.3 ± 12.3 | ns # | 46.0 ± 11.5 | ns # |
| LDL-C (mg/dL) | 100.6 ± 30.8 | 100.0 ± 29.2 | ns # | 102.1 ± 21.6 | ns # | 112.1 ± 47.1 | 103.6 ± 39.8 | ns # | 112.5 ± 45.7 | ns # |
| Triglycerides (mg/dL) | 154.5 ± 82.0 | 132.7 ± 69.8 | 0.0053 # | 147.0 ± 79.9 | 0.0084 # | 123.4 ± 63.7 | 120.4 ± 55.2 | ns # | 128.2 ± 55.2 | ns # |
| Sideremia (μg/dL) | 86.8 ± 34.2 | 73.6 ± 18.1 | ns # | 73.9 ± 17.6 | ns # | 75.5 ± 22.4 | 68.6 ± 20.4 | ns # | 75.0 ± 21.5 | ns # |
| Glycaemia (mg/dL) | 98.5 ± 25.3 | 96.7 ± 35.4 | ns # | 98.7 ± 37.3 | ns # | 93.4 ± 19.8 | 89.8 ± 13.2 | ns # | 89.2 ± 17.6 | ns # |
| Uric acid (mg/dL) | 6.7 ± 1.5 | 5.6 ± 1.2 | 0.0206 # | 6.1 ± 1.6 | ns # | 6.6 ± 1.4 | 6.0 ± 0.8 | 0.0426 # | 6.3 ± 1.6 | ns # |
| CRP (mg/L) | 4.2 ± 2.9 | 2.8 ± 2.3 | 0.0011 # | 2.7 ± 2.1 | ns # | 5.2 ± 4.5 | 3.0 ± 2.9 | 0.0070 # | 4.89 ± 4.1 | 0.0072 # |
| ESR (mm/h) | 41.6 ± 24.7 | 35.1 ± 20.5 | 0.0005 # | 43.9 ± 27.2 | 0.0218 # | 38.5 ± 29.1 | 30.2 ± 24.4 | 0.0063 # | 36.7 ± 27.7 | 0.0090 # |
| SYN | LUX | |
|---|---|---|
| p-Value (T0 vs. T2) | p-Value (T0 vs. T2) | |
| Creatinine (mg/dL) | ns # | ns # |
| e-GFR (mL/min/1.73 m2) | 0.0476 # | ns # |
| Albuminuria (mg/dL) | ns # | ns # |
| Albumin (g/dL) | 0.0041 # | ns # |
| Azotaemia (mg/dL) | 0.0389 # | ns # |
| Sodium (mEq/L) | ns # | ns # |
| Potassium (mEq/L) | ns # | ns # |
| Calcium (mg/dL) | ns # | ns # |
| Phosphorus (mg/dL) | ns # | ns # |
| TC (mg/dL) | ns # | ns # |
| HDL-C (mg/dL) | ns # | ns # |
| LDL-C (mg/dL) | ns # | ns # |
| Triglycerides (mg/dL) | ns # | ns # |
| Sideremia (μg/dL) | ns # | ns # |
| Glycaemia (mg/dL) | ns # | ns # |
| Uric acid (mg/dL) | 0.0230 # | ns # |
| CRP (mg/L) | 0.0007 # | ns # |
| ESR (mm/h) | ns # | ns # |
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | |
| FORT (U) | 289.3 ± 145.8 | 194.0 ± 63.1 | 0.00247 # | 223.3 ± 87.9 | ns # | 352.4 ± 156.5 | 278.6 ± 128.2 | ns # | 337.2 ± 143.7 | ns # |
| FORD (mmol/L Trolox) | 1.3 ± 0.4 | 1.2 ± 0.5 | ns # | 1.1 ± 0.4 | ns # | 1.3 ± 0.5 | 0.9 ± 0.5 | ns # | 1.5 ± 0.6 | ns # |
| IL-6 (pg/mL) | 85.2 ± 110.7 | 34.3 ± 11.1 | 0.0321 # | 42.8 ± 51.6 | ns # | 77.9 ± 98.3 | 59.8 ± 25.7 | ns # | 65.2 ± 34.2 | ns # |
| SYN | LUX | |
|---|---|---|
| p-Value (T0 vs. T2) | p-Value (T0 vs. T2) | |
| FORT (U) | ns # | ns # |
| FORD (mmol/L Trolox) | ns # | ns # |
| IL-6 (pg/mL) | 0.043 # | ns # |
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | T0 | T1 | p-Value (T0 vs. T1) | T2 | p-Value (T1 vs. T2) | |
| Weight (kg) | 79.1 ± 14.1 | 78.8 ± 13.8 | ns # | 78.4 ± 13.2 | ns # | 75.8 ± 15.8 | 75.6 ± 15.5 | ns # | 76.6 ± 15.0 | ns # |
| BMI (kg/m2) | 28.9 ± 3.7 | 28.8 ± 3.4 | ns # | 28.7 ± 3.7 | ns # | 27.5 ± 5.9 | 27.4 ± 5.8 | ns # | 27.8 ± 5.8 | ns # |
| Waist to hip ratio | 0.93 ± 0.07 | 0.92 ± 0.07 | ns # | 0.92 ± 0.06 | ns # | 0.96 ± 0.09 | 0.95 ± 0.09 | ns # | 0.95 ± 0.8 | ns # |
| Resistance (ohm) | 480.4 ± 87.3 | 450.8 ± 79.1 | ns # | 472.8 ± 96.9 | ns # | 498.7 ± 81.0 | 486.2 ± 98.2 | ns # | 496.0 ± 74.5 | ns # |
| Reactance (ohm) | 39.3 ± 10.8 | 42.3 ± 12.3 | 0.0391 # | 41.9 ± 11.3 | ns # | 41.8 ± 12.1 | 40.2 ± 10.0 | ns # | 41.8 ± 8.7 | ns # |
| Phase angle (°) | 4.6 ± 1.5 | 5.3 ± 2.2 | ns # | 4.8 ± 1.5 | ns # | 4.9 ± 0.5 | 5.4 ± 0.7 | ns # | 4.9 ± 0.5 | ns # |
| TBW (%) | 52.8 ± 6.1 | 55.1 ± 5.9 | 0.0465 # | 53.2 ± 6.3 | ns # | 56.3 ± 7.4 | 60.8 ± 8.3 | 0.0033 # | 54.4 ± 7.4 | 0.0301 # |
| ICW (%) | 46.8 ± 7.2 | 49.9 ± 8.7 | 0.0058 # | 47.0 ± 9.0 | ns # | 48.5 ± 5.0 | 49.5 ± 6.3 | ns # | 45.1 ± 14.4 | ns # |
| ECW (%) | 53.2 ± 7.2 | 50.1 ± 8.7 | 0.0058 # | 53.0 ± 8.8 | ns # | 51.5 ± 3.1 | 50.5 ± 3.5 | ns # | 54.9 ± 3.0 | ns # |
| FM (%) | 34.4 ± 8.2 | 31.5 ± 8.2 | ns # | 32.6 ± 8.0 | ns # | 28.6 ± 9.6 | 22.2 ± 10.1 | ns # | 26.5 ± 8.5 | ns # |
| FFM (%) | 65.6 ± 7.6 | 68.5 ± 8.2 | ns # | 67.4 ± 7.9 | ns # | 71.4 ± 9.6 | 77.8 ± 10.5 | ns # | 73.5 ± 9.9 | ns # |
| MM (%) | 38.3 ± 6.5 | 41.6 ± 7.2 | 0.0198 # | 40.7 ± 7.5 | ns # | 43.9 ± 6.7 | 45.5 ± 5.9 | ns # | 44.3 ± 706 | ns # |
| BCMI (kg/m2) | 9.1 ± 2.7 | 10.2 ± 3.6 | 0.0230 # | 9.1 ± 2.9 | 0.0296 # | 8.9 ± 1.7 | 9.7 ± 2.3 | ns # | 8.3 ± 2.9 | ns # |
| SYN | LUX | |
|---|---|---|
| p-Value (T0 vs. T2) | p-Value (T0 vs. T2) | |
| Weight (kg) | ns # | ns # |
| BMI (kg/m2) | ns # | ns # |
| Waist/hip ratio | ns # | ns # |
| Resistance (ohm) | ns # | ns # |
| Reactance (ohm) | 0.0485 # | ns # |
| Phase angle (°) | ns # | ns # |
| TBW (%) | ns # | ns # |
| ICW (%) | ns # | ns # |
| ECW (%) | ns # | ns # |
| FM (%) | ns # | ns # |
| FFM (%) | ns # | ns # |
| MM (%) | 0.0400 # | ns # |
| BCMI (kg/m2) | ns # | ns # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020581
Noce A, Marrone G, Urciuoli S, Di Daniele F, Di Lauro M, Pietroboni Zaitseva A, Di Daniele N, Romani A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients. 2021; 13(2):581. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020581
Chicago/Turabian StyleNoce, Annalisa, Giulia Marrone, Silvia Urciuoli, Francesca Di Daniele, Manuela Di Lauro, Anna Pietroboni Zaitseva, Nicola Di Daniele, and Annalisa Romani. 2021. "Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients" Nutrients 13, no. 2: 581. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020581