Multinutrient Biofortification of Maize (Zea mays L.) in Africa: Current Status, Opportunities and Limitations
Abstract
:1. Introduction
2. Maize Uses, Malnutrition Prevalence and Health Risks of Maize-Based Diets in SSA
2.1. Maize Is “Life” in Africa
2.2. Prevalence and Impact of Zn, Provitamin A and Protein Deficiency in SSA
2.3. Dietary Reference Intake
2.4. Physiological Functions of Vitamin A, Zinc, Lysine and Tryptophan
3. Strategies to Alleviate Vitamin A, Zinc and Protein Malnutrition
3.1. Industrial Fortification
3.2. Pharmaceutical Supplementation
3.3. Dietary Diversification
3.4. Agronomic Practices
3.5. Genetic Improvement of Maize for Zn, Provitamin A and Quality Protein
3.5.1. QPM Genetics and Breeding History
3.5.2. Provitamin A Maize and Major Carotenoids in Maize Grain
3.5.3. Genetic Basis for High Kernel Zn Content in Maize
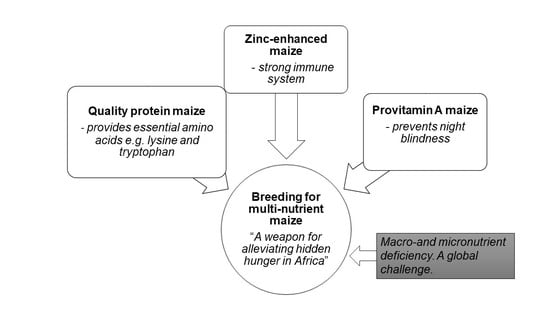
4. Breeding Strategies for Multinutrient Biofortified Maize
4.1. Making Use of the Existing Genetic Variability in Maize Germplasm
4.2. Germplasm Introductions and Testing for Stability in Local Environments
4.3. Exploiting Heterosis through Hybridization
4.4. Marker-Assisted Breeding
4.5. Mutation Breeding
4.6. Use of Transgenics in Developing Multinutrient Maize
4.7. Genome Editing
5. Major Challenges in Developing Multinutrient Maize
5.1. Acceptance of Multinutrient Maize in a QPM Background
5.2. Acceptance of Multinutrient Maize in a Provitamin A Background
5.3. Acceptance of Multinutrient Maize on Zn Genetic Background
5.4. Low Yield Potential of Biofortified Maize Cultivars
5.5. Quality Assurance for Multinutrient Maize
5.6. Policy Regulations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindu, V.; Palacios-Rojas, N.; Babu, R.; Suwarno, W.B.; Rashid, Z.; Usha, R.; Saykhedkar, G.R.; Nair, S.K. Identification and validation of genomic regions influencing kernel zinc and iron in maize. Theor. Appl. Genet. 2018, 131, 1443–1457. [Google Scholar] [CrossRef] [Green Version]
- Schmidhuber, J.; Sur, P.; Fay, K.; Huntley, B.; Salama, J.; Lee, A.; Cornaby, L.; Horino, M.; Murray, C.; Afshin, A. The Global Nutrient Database: Availability of macronutrients and micronutrients in 195 countries from 1980 to 2013. Lancet Planet Health 2018, 2, E353–E368. [Google Scholar] [CrossRef] [Green Version]
- Menkir, A. Genetic variation for grain mineral content in tropical-adapted maize inbred lines. Food Chem. 2008, 110, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maziya-Dixon, B.; Kling, J.G.; Menkir, A.; Dixon, A. Genetic variation in total carotene, iron, and zinc contents of maize and cassava genotypes. Food Nutr. Bull. 2000, 21, 419–422. [Google Scholar] [CrossRef]
- Jin, T.; Zhou, J.; Chen, J.; Zhu, L.; Zhao, Y.; Huang, Y. The genetic architecture of zinc and iron content in maize grains as revealed by QTL mapping and meta-analysis. Breed. Sci. 2013, 63, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Nuss, E.T.; Tanumihardjo, S.A. Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2011, 2, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, K.; Siwela, M.; Derera, J.; Veldman, F.J. Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods. J. Food Sci. Technol. 2014, 51, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Bänziger, M.; Long, J. The potential for increasing the iron and zinc density of maize through plant breeding. Food Nutr. Bull. 2000, 21, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Halilu, A.D.; Shehu, G.A.; Daniel, A.A.; Usman, I.S. Genetics of carotenoids for provitamin A biofortification in tropical-adapted maize. Crop J. 2016, 4, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, Z.; Rouached, H.; Rakha, A. Combating mineral malnutrition through iron and zinc biofortification of cereals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 329–346. [Google Scholar] [CrossRef]
- Hefferon, K.L. Nutritionally enhanced food crops; progress and perspectives. Int. J. Mol. Sci. 2015, 16, 3895–3914. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Vasal, S.K.; Kassahun, B.; Singh, N.N. Quality protein maize. Curr. Sci. 2001, 81, 1308–1319. [Google Scholar]
- Brookie, K.L.; Best, G.I.; Conner, T.S. Intake of raw fruits and vegetables is associated with better mental health than intake of processed fruits and vegetables. Front. Psyc. 2018, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Duvick, D.N. Post–green revolution trends in yield potential of temperate maize in the North-Central United States. In Agronomy and Horticulture–Faculty Publications 96; University of Nebraska: Nebraska, NE, USA, 1999. [Google Scholar]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A. Sub-Saharan African maize-based foods: Technological perspectives to increase the food and nutrition security impacts of maize breeding programmes. Glob. Food Sec. 2019, 17, 48–56. [Google Scholar] [CrossRef]
- Muzhingi, T.; Gadaga, T.H.; Siwela, A.H.; Grusak, M.A.; Russell, R.M.; Tang, G. Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am. J. Clin. Nutr. 2011, 94, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; San Vicente, F.; Nair, S.K.; Vivek, B.S.; et al. Molecular breeding for nutritionally enriched maize: Status and prospects. Front. Gen. 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makumbi, D.; Betrán, J.F.; Bänziger, M.; Ribaut, J.M. Combining ability, heterosis and genetic diversity in tropical maize (Zea mays L.) under stress and non-stress conditions. Euphytica 2011, 180, 143–162. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Sec. 2013, 5, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Ertiro, B.T.; Beyene, Y.; Das, B.; Mugo, S.; Olsen, M.; Oikeh, S.; Juma, C.; Labuschagne, M.; Prasanna, B.M. Combining ability and testcross performance of drought-tolerant maize inbred lines under stress and non-stress environments in Kenya. Plant Breed. 2017, 136, 197–205. [Google Scholar] [CrossRef]
- Santpoort, R. The drivers of maize area expansion in sub-Saharan Africa. How policies to boost maize production overlook the interests of smallholder farmers. Land 2020, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Ranum, P.; Peña-Rosas, P.J.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Oikeh, S.O.; Menkir, A.; Maziya-Dixon, B.; Welch, R.; Glahn, R.P. Assessment of concentrations of iron and zinc and bioavailable iron in grains of early-maturing tropical maize varieties. J. Agric. Food Chem. 2003, 51, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Rampure, M.; Todkar, A.; Sharma, P. Ethanol from maize: An entrepreneurial opportunity in agro-business. Biofuels 2019, 10, 385–391. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Morawetz, U.B. Estimating consumer willingness to pay for food quality with experimental auctions: The case of yellow versus fortified maize meal in Kenya. Agric. Econ. 2011, 42, 1–16. [Google Scholar] [CrossRef]
- Harika, R.; Faber, M.; Samuel, F.; Kimiywe, J.; Mulugeta, A.; Eilander, A. Are low intakes and deficiencies in iron, vitamin A, zinc, and iodine of public health concern in Ethiopian, Kenyan, Nigerian, and South African children and adolescents? Food Nutr. Bull. 2017, 38, 405–427. [Google Scholar] [CrossRef]
- Lividini, K.; Fiedler, J.L. Assessing the promise of biofortification: A case study of high provitamin A maize in Zambia. Food Policy 2015, 54, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Brazier, A.K.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Global Health 2015, 3, e528–e536. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, A.M.; Abdel Aleem, M.M.; El-Shazly, A.A. Maternal vitamin a deficiency during pregnancy and its relation with maternal and neonatal hemoglobin concentrations among poor Egyptian families. ISRN Ped. 2013, 2013, 652148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D. The rise and fall of protein malnutrition in global health. Ann. Nutr. Met. 2016, 69, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Ass. J. 2005, 173, 3. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.A.; Aslam, M.; Beshir, A.; Khan, M.S. Breeding for provitamin A biofortification of maize (Zea mays L.). Plant Breed. 2018, 137, 451–469. [Google Scholar] [CrossRef]
- Andersson, M.S.; Saltzman, A.; Virk, P.S.; Pfeiffer, W.H. Progress update: Crop development of biofortified staple food crops under Harvestplus. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11905–11935. [Google Scholar] [CrossRef]
- Rafii, M.; Elango, R.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Metabolic availability of the limiting amino acids lysine and tryptophan in cooked white African corn meal assessed in healthy young men using the indicator amino acid oxidation technique. J. Nutr. 2018, 148, 917–924. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir, A. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Manjeru, P.; van Biljon, A.; Labuschagne, M. The development and release of maize fortified with provitamin A carotenoids in developing countries. Crit. Rev. Food Sci. Nutr. 2019, 59, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Harjes, C.E.; Rocheford, T.R.; Bai, L.; Brutnell, T.P.; Kandianis, C.B.; Sowinski, S.G.; Stapleton, A.E.; Vallabhaneni, R.; Williams, M.; Wurtzel, E.T.; et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 2008, 319, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Op. Gastroent. 2009, 25, 136–143. [Google Scholar] [CrossRef]
- Kurita, H.; Ohsako, S.; Hashimoto, S.; Yoshinaga, J.; Tohyama, C. Prenatal zinc deficiency-dependent epigenetic alterations of mouse metallothionein-2 gene. J. Nutr. Biochem. 2012, 24, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Knoell, D.L.; Julian, M.W.; Bao, S.; Besecker, B.; Macre, J.E.; Leikauf, G.D.; DiSilvestro, A.R.; Crouser, E.D. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit. Care Med. 2009, 37, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.S.; Hossain, F.; Muthusamy, V. Biofortification of maize: An Indian perspective. Ind. J. Genet. Plant Breed. 2015, 75, 1–22. [Google Scholar] [CrossRef]
- Kipsang, J.K.; Choge, J.K.; Marinda, P.A.; Khayeka-Wandabwa, C. Pellagra in isoniazid preventive and antiretroviral therapy. IDCases 2019, 17, e00550. [Google Scholar] [CrossRef]
- Murgia, I.; De Gara, L.; Grusak, M.A. Biofortification: How can we exploit plant science and biotechnology to reduce micronutrient deficiencies? Front. Plant Sci. 2013, 4, 429. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar] [PubMed]
- Lalani, B.; Bechoff, A.; Bennett, B. Which choice of delivery model (s) works best to deliver fortified foods? Nutrients 2019, 11, 1594. [Google Scholar] [CrossRef] [Green Version]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified crops for tackling micronutrient deficiencies–what impact are these having in developing countries and could they be of relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Eichler, K.; Wieser, S.; Ruthemann, I.; Brugger, U. Effects of micronutrient fortified milk and cereal food for infants and children: A systematic review. BMC Public Health 2012, 12, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomo, Z.A.R.; Allain, T.J.; Matenga, J.A.; Ndemere, B.; Wilson, A.; Urdal, P. Urinary iodine concentrations and thyroid function in adult Zimbabweans during a period of transition in iodine status. Am. J. Clin. Nutr. 1999, 70, 888–891. [Google Scholar] [CrossRef] [Green Version]
- Omole, J.O.; Ighodaro, O.M.; Durosinolorun, O. Fortification of Ogi with whey increases essential amino acids content of fortified product. Int. Schol. Res. Not. 2017, 2017, 7450845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, S.Y.; Brown, K.H. Impact of zinc fortification on zinc nutrition. Food Nutr. Bull. 2009, 30, S79–S107. [Google Scholar] [CrossRef]
- Yusufali, R.; Sunley, N.; de Hoop, M.; Panagides, D. Flour fortification in South Africa: Post-implementation survey of micronutrient levels at point of retail. Food Nutr. Bull. 2012, 33, S321–S329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bégin, F.; Cervinskas, J.; Mannar, V. Food fortification with vitamin A: The potential for contributing to the elimination of vitamin A deficiency in Africa. Food Nutr. Bull. 2001, 22, 408–415. [Google Scholar] [CrossRef]
- Brown, K.H.; Peerson, J.M.; Baker, S.K.; Hess, S.Y. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr. Bull. 2009, 30, S12–S40. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C. International zinc nutrition consultative group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. Int. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Sakulchit, T.; Goldman, R.D. Zinc supplementation for paediatric pneumonia. Can. Fam. Phys. 2017, 63, 763–765. [Google Scholar]
- Bhutta, Z.A.; Salam, R.A.; Das, J.K. Meeting the challenges of micronutrient malnutrition in the developing world. Brit. Med. Bull. 2013, 106, 7–17. [Google Scholar] [CrossRef]
- Das, J.K.; Kumar, R.; Salam, R.A.; Bhutta, Z.A. Systematic review of zinc fortification trials. Ann. Nutr. Metab. 2013, 62, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dairo, M.D.; Ige, O.K. Supplementation of micronutrient in community micronutrient deficiency prevention programmes. Ann. Ibadan Postgrad. Med. 2009, 7, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Shah, N.A. Vitamin A Toxicity; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK532916/ (accessed on 15 October 2020).
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Gilani, G.S.; Nasim, A. Impact of foods nutritionally enhanced through biotechnology in alleviating malnutrition in developing countries. JAOACI 2007, 90, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; Anderson, V.P. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr. Bull. 2009, 30, S108–S143. [Google Scholar] [CrossRef]
- Lim, K.H.C.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Aakre, I.; Bøkevoll, A.; Chaira, J.; Bouthir, F.Z.; Frantzen, S.; Kausland, A.; Kjellevold, M. Variation in nutrient composition of seafood from North West Africa: Implications for food and nutrition security. Foods 2020, 9, 1516. [Google Scholar] [CrossRef]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manwaring, H.R.; Bligh, H.F.J.; Yadav, R. The challenges and opportunities associated with biofortification of pearl millet (Pennisetum glaucum) with elevated levels of grain iron and zinc. Front. Plant Sci. 2016, 7, 1944. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; Ferguson, E.L. Assessment of dietary zinc in a population. Am. J. Clin. Nutr. 1998, 68, 430S–434S. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.J. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion–evidence in humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S. [Google Scholar] [CrossRef] [Green Version]
- Chilungo, S.; Muzhingi, T.; Van-Den, T.; Allen, C.J. Effect of storage and packaging materials on colour and carotenoid content of orange-fleshed sweet potato flours. Int. J. Innov. Sci. Res. Technol. 2019, 4, 362–369. [Google Scholar]
- Purushottam, S.K.S.; Uddeen, R. Nutri-farms for mitigating malnutrition in India. In Biofortification of Food Crops; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New Delhi, India, 2016; pp. 461–477. [Google Scholar]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic approach of zinc biofortification can increase zinc bioavailability in wheat flour and thereby reduce zinc deficiency in humans. Nutrients 2017, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Ruel, M.T.; Bouis, H.E. Plant breeding: A long term strategy for the control of Zn deficiency in vulnerable populations. Am. J. Clin. Nutr. 1998, 68, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.; Welch, R. Biofortification–A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Machida, L.; Derera, J.; Tongoona, P.; MacRobert, J. Combining ability and reciprocal cross effects of elite quality protein maize inbred lines in subtropical environments. Crop Sci. 2010, 50, 1708–1717. [Google Scholar] [CrossRef]
- Pixley, K.; Palacios, N.R.; Babu, R.; Mutale, R.; Surles, R.; Simpungwe, E. Biofortification of maize with provitamin A carotenoids. In Carotenoids in Human Health; Tanumihardjo, S.A., Ed.; Springer Science and Business Media: New York, NY, USA, 2013; pp. 271–292. [Google Scholar]
- Chomba, E.; Westcott, C.M.; Westcott, J.E.; Mpabalwani, E.M.; Krebs, N.F.; Patinkin, Z.W.; Palacios, N.; Hambidge, K. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J. Nutr. 2015, 45, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuma, M.K.; Kolanisi, U.; Modi, A.T. The potential of integrating provitamin A-biofortified maize in smallholder farming systems to reduce malnourishment in South Africa. Env. Res. Public Health 2018, 15, 805. [Google Scholar] [CrossRef] [Green Version]
- Mugode, L.; Ha, B.; Kaunda, A.; Sikombe, T.; Phiri, S.; Mutale, R.; Davis, C.; Tanumihardjo, S.; De Moura, F.F. Carotenoid retention of biofortified maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage. J. Agric. Food Chem. 2014, 62, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Nkhata, S.G.; Chilungo, S.; Memba, A.; Mponela, P. Biofortification of maize and sweetpotatoes with provitamin A carotenoids and implication on eradicating vitamin A deficiency in developing countries. J. Agric. Food Res. 2020, 2, 100068. [Google Scholar] [CrossRef]
- Nyakurwa, C.S.; Gasura, E.; Mabasa, S. Potential for quality protein maize for reducing protein energy undernutrition in maize dependent sub-Saharan African countries: A review. Afr. Crop Sci. J. 2017, 25, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Mebratu, A.; Wegary, D.; Mohammed, W.; Teklewold, A.; Tarekegne, A. Genotype environment interaction of quality protein maize hybrids under contrasting management conditions in eastern and southern Africa. Crop Sci. 2019, 59, 1576–1589. [Google Scholar] [CrossRef] [Green Version]
- Nedi, G.; Alamerew, S.; Tulu, L. Review on quality protein maize breeding for Ethiopia. J. Biol. Agric. Healthc. 2016, 6, 84–96. [Google Scholar]
- Twumasi-Afriye, S.; Palacios Rojas, N.; Friesen, D.; Teklewold, A.; Gissa, D.W.; De Groote, H.; Prasanna, B.M. Guidelines for the Quality Control of Quality Protein Maize (QPM) Seed and Grain: Technical Bulletin; CGIAR; CIMMYT: Addis Ababa, Ethiopia, 2016; p. 38. [Google Scholar]
- Vasal, S.K. The quality protein maize story. Food Nutr. Bull. 2000, 21, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Babu, R.; Nair, S.; Kumar, A.; Venkatesh, S.; Shekhar, J.; Singh, N.N.; Gupta, H. Two generation marker aided backcrossing for rapid conversion of normal maize lines to quality protein maize. Theor. Appl. Genet. 2005, 111, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Vivek, B.S.; Krivanek, A.F.; Palacios-Rojas, N.; Twumasi-Afriyie, S.; Diallo, A.O. Breeding Quality Protein Maize (QPM): Protocols for Developing QPM Cultivars; CIMMYT: El Batán, Mexico, 2008. [Google Scholar]
- Teklewold, A.; Wegary, D.; Tadesse, A.; Tadesse, B.; Bantte, K.; Friesen, D.; Prasanna, B.M. Quality Protein Maize (QPM): A Guide to the Technology and its Promotion in Ethiopia; CIMMYT: Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Graham, G.G.; Glover, D.V.; de Romana, G.L.; Morales, E.; MacLean, W.C. Nutritional value of normal, opaque-2 and sugary-2 opaque-2 maize hybrids for infants and children. I: Digestibility and utilization. J. Nutr. 1980, 110, 1061–1069. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Sandmann, G.; Capell, T.; Christou, P.; Zhu, C. The silencing of carotenoid β-hydroxylases by RNA interference in different maize genetic backgrounds increases the β-carotene content of the endosperm. Int. J. Mol. Sci. 2017, 18, 2515. [Google Scholar] [CrossRef] [Green Version]
- Wurtzel, E.T.; Cuttriss, A.; Vallabhaneni, R. Maize provitamin A carotenoids, current resources, and future metabolic engineering challenges. Front. Plant Sci. 2012, 3, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toti, E.; Chen, C.O.; Palmery, M.; Villano Valencia, D.; Peluso, I. Non-provitamin A and provitamin A carotenoid as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxid. Med. Cell. Long. 2018, 2018, 4637861. [Google Scholar] [CrossRef] [PubMed]
- Simpungwe, E.; Dhliwayo, T.; Palenberg, M.; Taleon, V.; Birol, E.; Oparinde, A.; Saltzman, A.; Diressie, M.T. Orange maize in Zambia: Crop development and delivery experience. Afr. J. Food Agric. Nutr. 2017, 17, 11973–11999. [Google Scholar] [CrossRef]
- Chakraborti, M.; Prasanna, B.M.; Hossain, F.; Mazumdar, S.; Singh, A.M.; Guleria, S.; Gupta, H.S. Identification of kernel iron- and zinc-rich maize inbreds and analysis of genetic diversity using microsatellite markers. J. Plant Biochem. Biotechnol. 2011, 20, 224–233. [Google Scholar] [CrossRef]
- Ghandilyan, A.; Vreugdenhil, D.; Aarts, M.G.M. Progress in the genetic understanding of plant iron and zinc. Phys. Plant. 2006, 126, 407–417. [Google Scholar] [CrossRef]
- Anuradha, K.; Agarwal, S.; Rao, Y.V.; Rao, K.V.; Viraktamath, B.C.; Sarla, N. Mapping QTLs and candidate genes for iron and zinc concentrations in unpolished rice of Madhukar X Swarna RILs. Gene 2012, 508, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chauhan, R.S. Identification of candidate gene-based markers (SNPs and SSRs) in the zinc and iron transporter sequences of maize (Zea mays L.). Curr. Sci. 2008, 95, 8–25. [Google Scholar]
- Qin, H.; Cai, Y.; Liu, Z.; Wang, G.; Wang, J.; Guo, Y.; Wang, H. Identification of QTL for zinc and iron concentration in maize kernel and cob. Euphytica 2012, 187, 345–358. [Google Scholar] [CrossRef]
- Queiroz-Queiroz, V.A.V.; Guimarães, P.E.O.; Queiroz, L.R.; Guedes, E.O.; Vasconcelos, V.D.B.; Guimarães, L.J.; Ribeiro, P.E.A.; Schaffert, R.E. Iron and zinc availability in maize lines. Ciência Tecn. Alimen. 2011, 31, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Mageto, E.K.; Lee, M.; Dhliwayo, T.; Palacios-Rojas, N.; Vicente, F.S.; Burgueño, J.; Hallauer, A.R. An evaluation of kernel zinc in hybrids of elite quality protein maize (QPM) and non-QPM inbred lines adapted to the tropics based on a mating design. Agronomy 2020, 10, 695. [Google Scholar]
- Garcia-Oliveira, A.L.; Chander, S.; Ortiz, R.; Menkir, A.; Gedil, M. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front. Plant Sci. 2018, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Goudia, B.D.; Hash, C.T. Breeding for high grain Fe and Zn levels in cereals. Int. J. Innov. Appl. Stud. 2015, 12, 342–354. [Google Scholar]
- Egesel, C.O.; Wong, J.C.; Lambert, R.J.; Rocheford, T.R. Combining ability of maize inbreds for carotenoids and tocopherols. Crop Sci. 2003, 43, 818–823. [Google Scholar] [CrossRef]
- De Groote, H.; Gunaratna, N.; Ergano, K.; Friesen, D. Extension and adoption of biofortified crops: Quality protein maize in East Africa. In Proceedings of the Joint 3rd African Association of Agricultural Economists (AAAE) and 48th Agricultural Economists Association of South Africa (AEASA) Conference, Cape Town, South Africa, 19–23 September 2010. [Google Scholar]
- Prasanna, B.M.; Mazumdar, S.; Chakraborti, M.; Hossain, F.; Manjaiah, K.M.; Agrawal, P.K. Genetic variability and genotype x environment interactions for kernel Fe and Zn concentrations in maize (Zea mays) genotypes. Ind. J. Agric. Sci. 2011, 81, 704–711. [Google Scholar]
- Chakraborti, M.; Hossain, F.; Kumar, R.; Gupta, H.S.; Prasanna, B.M. Genetic evaluation of grain yield and kernel micronutrient traits in maize. Pusa Agric. Sci. 2009, 32, 11–16. [Google Scholar]
- Suwarno, W.B.; Pixley, K.V.; Palacios-Rojas, N.; Kaeppler, S.M.; Babu, R. Formation of heterotic groups and understanding genetic effects in a provitamin a biofortified maize breeding program. Crop Sci. 2014, 54, 14–24. [Google Scholar] [CrossRef]
- Chakraborti, M.; Prasanna, B.M.; Singh, A.; Hossain, F. Generation mean analysis of kernel iron and zinc concentrations in maize (Zea mays L.). Ind. J. Agric. Sci. 2010, 80, 956–959. [Google Scholar]
- Galluzzi, G.; Halewood, M.; Noriega, I.L.; Vernooy, R. Twenty-five years of international exchanges of plant genetic resources facilitated by the CGIAR genebanks: A case study on global interdependence. Biodiv. Cons. 2016, 25, 1421–1446. [Google Scholar] [CrossRef] [Green Version]
- Masuka, B.; Magorokosho, C.; Olsen, M.; Atlin, G.N.; Bänziger, M.; Pixley, K.V.; Vivek, B.S.; Labuschagne, M.; Matemba-Mutasa, R.; Burgueño, J.; et al. Gains in maize genetic improvement in eastern and southern Africa: II. CIMMYT open-pollinated variety breeding pipeline. Crop Sci. 2017, 57, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Pixley, K.; Bänziger, M. Open-pollinated varieties: A backward step or valuable option for farmers. In Integrated Approaches to Higher Maize Productivity in the New Millennium; Friesen, D.K., Palmer, A.F.E., Eds.; CIMMYT and KARI: Nairobi City, Kenya, 2002; pp. 22–28. [Google Scholar]
- Warburton, M.L.; Rauf, S.; Marek, L.; Hussain, M.; Ogunola, O.; Gonzalez, J.S. The use of crop wild relatives in maize and sunflower breeding. Crop Sci. 2017, 57, 1227–1240. [Google Scholar] [CrossRef]
- Okai, D.B.; Boateng, M.; Ewool, M.B.; Ankamaa, D.; Osarumwense, S.O. Nutritional evaluation of some new maize varieties: Effects on growth performance and carcass traits of albino rats. Afr. J. Food Agric. Dev. 2015, 15, 10306–10316. [Google Scholar]
- Duvick, D.N. Genetic progress in yield of United States maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Kassie, G.T.; Erenstein, O.; Mwangi, W.; La Rovere, R.; Setimela, P.; Langyintuo, A. Characterization of Maize Production in Southern Africa: Synthesis of CIMMYT/DTMA Household Level Farming System Surveys in Angola, Malawi, Mozambique, Zambia and Zimbabwe; Socio-Economics Program Working Paper 4; CIMMYT: El Batán, Mexico, 2012. [Google Scholar]
- Long, J.K.; Bänziger, M.; Smith, M.E. Diallel analysis of grain iron and zinc density in Southern African adapted maize inbreds. Crop Sci. 2004, 44, 2019–2026. [Google Scholar] [CrossRef]
- Babu, R.; Rojas, N.P.; Gao, S.; Yan, J.; Pixley, K. Validation of the effects of molecular marker polymorphisms in LcyE and CrtRB1 on provitamin A concentrations for 26 tropical maize populations. Theor. Appl. Genet. 2013, 126, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, P.A.; Wani, S.A.; Rather, A.G.; Wani, S.H. Quality protein maize (QPM): Genetic manipulation for the nutritional fortification of maize. J. Plant Breed. Crop Sci. 2009, 1, 244–253. [Google Scholar]
- Liu, L.; Jeffers, D.; Zhang, Y.D.; Ding, M.L.; Chen, W.; Kang, M.S.; Fan, X. Introgression of the crtRB1 gene into quality protein maize inbred lines using molecular markers. Mol. Breed. 2015, 35, 154. [Google Scholar] [CrossRef] [Green Version]
- Zunjare, R.U.; Hossain, F.; Muthusamy, V.; Baveja, A.; Chauhan, H.S.; Bhat, J.S.; Thirunavukkarasu, N.; Saha, S.; Gupta, H.S. Development of biofortified maize hybrids through marker-assisted stacking of β-carotene hydroxylase, lycopene-ε-cyclase and opaque2 genes. Front. Plant Sci. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.M.; Manicacci, D.; Falque, M.; Damerval, C. Molecular evolution of the opaque-2 gene in Zea mays L. J. Mol. Evol. 2005, 61, 551–558. [Google Scholar] [CrossRef]
- Tandzi, L.N.; Mutengwa, C.S.; Ngonkeu, E.L.M.; Woïn, N.; Gracen, V. Breeding for Quality Protein Maize (QPM) varieties: A Review. Agronomy 2017, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Efron, Y. An EMS-sensitivity factor in maize conditioning albino leaf stripes. Genetics 1974, 78, 399–867. [Google Scholar] [CrossRef]
- Tadele, Z. Mutagenesis and TILLING to dissect gene function in plants. Cur. Genom. 2016, 17, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, M.P.; Pollak, L.M. Transgenic maize. Starch 2005, 57, 187–195. [Google Scholar] [CrossRef]
- Tang, M.; He, X.; Luo, Y.; Ma, L.; Tang, X.; Huang, K. Nutritional assessment of transgenic lysine-rich maize compared with conventional quality protein maize. J. Sci. Food Agric. 2013, 93, 1049–1054. [Google Scholar] [CrossRef]
- Zimmermann, R.; Qaim, M. Potential health benefits of golden rice: A Philippine case study. Food Policy 2004, 29, 147–168. [Google Scholar] [CrossRef]
- Aluru, M.; Yang, X.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin A content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.; Cao, J.; Lim, Y.; Arato, T.; Kodama, K. Exploring factors affecting the acceptance of genetically edited food among youth in Japan. Int. J. Environ. Res. Publ. 2020, 17, 2935. [Google Scholar] [CrossRef]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic biofortification to enrich rice and wheat grain iron: From genes to product. Front. Plant Sci. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Alok, A.; Shivani, K.P.; Kaur, N.; Awasthi, P.; Chaturvedi, S.; Pandey, P.; Pandey, A.K.; Pandey, A.; Tiwari, S. CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Met. Eng. 2020, 59, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant genome engineering for targeted improvement of crop traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Tessema, M.; Gunaratna, N.S.; Donato, K.; Cohen, J.L.; McConnell, M.; Belayneh, D.; Brouwer, I.D.; Belachew, T.; De Groote, H. Translating the impact of quality protein maize into improved nutritional status for Ethiopian children: Study protocol for a randomized controlled trial. BMC Nutr. 2016, 2, 54. [Google Scholar] [CrossRef] [Green Version]
- Nuss, E.T.; Arscott, S.A.; Bresnahan, K.; Pixley, K.V.; Rocheford, T.; Hotz, C.; Siamusantu, W.; Chileshe, J.; Tanumihardjo, S.A. Comparative intake of white-versus orange colored maize by Zambian children in the context of promotion of biofortified maize. Food Nutr. Bull. 2012, 33, 63–71. [Google Scholar] [CrossRef]
- Muzhingi, T.; Langyintuo, A.S.; Malaba, L.C.; Bänziger, M. Consumer acceptability of yellow maize products in Zimbabwe. Food Policy 2008, 33, 352–361. [Google Scholar] [CrossRef]
- Meenakshi, J.V.; Banerji, A.; Manyong, V.; Tomlins, K.; Mittal, N.; Hamukwala, P. Using a discrete choice experiment to elicit the demand for a nutritious food: Willingness–to–pay for orange maize in rural Zambia. J. Health Econ. 2012, 31, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groote, H.; Kimenju, S.C. Consumer preferences for maize products in urban Kenya. Food Nutr. Bull. 2012, 33, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Derera, J.; Siwela, M.; Veldman, F.J. Consumer acceptance of yellow, provitamin A-biofortified maize in KwaZulu-Natal. South Afr. J. Clin. Nutr. 2011, 24, 186–191. [Google Scholar] [CrossRef]
- Taleon, V.; Mugode, L.; Cabrera-Soto, L.; Palacios-Rojas, N. Carotenoid retention in biofortified maize using different post-harvest storage and packaging methods. Food Chem. 2017, 232, 60–66. [Google Scholar] [CrossRef]
- Vaiknoras, K.; Larochelle, C.; Birol, E.; Asare-Marfo, D.; Herrington, C. Promoting rapid and sustained adoption of biofortified crops: What we learned from iron-biofortified bean delivery approaches in Rwanda. Food Policy 2019, 83, 271–284. [Google Scholar] [CrossRef]


| Risk Factor | DALYs (millions) | Risk Factor | DALYs (millions) |
|---|---|---|---|
| World | Low-income countries | ||
| Underweight | 91 | Underweight | 82 |
| Unsafe sex | 70 | Unsafe water | 53 |
| Alcohol | 69 | Unsafe sex | 52 |
| Unsafe water | 64 | Suboptimal breastfeeding | 34 |
| Blood pressure | 57 | Indoor smoking | 33 |
| Tobacco use | 57 | Vitamin A deficiency | 20 |
| Suboptimal breastfeeding | 44 | Blood pressure | 18 |
| High blood glucose | 41 | Alcohol | 18 |
| Indoor smoking | 41 | High blood glucose | 16 |
| Obesity | 36 | Zinc deficiency | 14 |
| Variety | Target Trait | Target Countries | Year of Release | Reference |
|---|---|---|---|---|
| BIO-MZN01 | Zinc | Columbia | 2018 | [1] |
| ICTA HB-15 | Zinc | Guatemala | 2018 | [40] |
| ICTA B-15 | Zinc | Guatemala | 2018 | [40] |
| GV665A | Provitamin A | Zambia | 2012 | [87] |
| GV662A | Provitamin A | Zambia | 2012 | [88] |
| Abontem | Provitamin A | Ghana | 2012 | [89] |
| MH39A, MH40A | Provitamin A | Malawi | 2016 | [89] |
| ZS242A | Provitamin A | Zimbabwe | 2015 | [86] |
| RAHA02 | Provitamin A | Rwanda | 2017 | [89] |
| HQPM-5 | QPM | India | 2007 | [46] |
| Obatanpa | QPM | Ghana | 1992 | [90] |
| ZS261 | QPM | Zimbabwe | 2006 | [91] |
| BHQP542 | QPM | Ethiopia | 2001 | [92] |
| Q623 | QPM | South Africa | 2014 | [93] |
| Yanrui-1 | QPM | China | 2010 | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goredema-Matongera, N.; Ndhlela, T.; Magorokosho, C.; Kamutando, C.N.; van Biljon, A.; Labuschagne, M. Multinutrient Biofortification of Maize (Zea mays L.) in Africa: Current Status, Opportunities and Limitations. Nutrients 2021, 13, 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13031039
Goredema-Matongera N, Ndhlela T, Magorokosho C, Kamutando CN, van Biljon A, Labuschagne M. Multinutrient Biofortification of Maize (Zea mays L.) in Africa: Current Status, Opportunities and Limitations. Nutrients. 2021; 13(3):1039. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13031039
Chicago/Turabian StyleGoredema-Matongera, Nakai, Thokozile Ndhlela, Cosmos Magorokosho, Casper N. Kamutando, Angeline van Biljon, and Maryke Labuschagne. 2021. "Multinutrient Biofortification of Maize (Zea mays L.) in Africa: Current Status, Opportunities and Limitations" Nutrients 13, no. 3: 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13031039