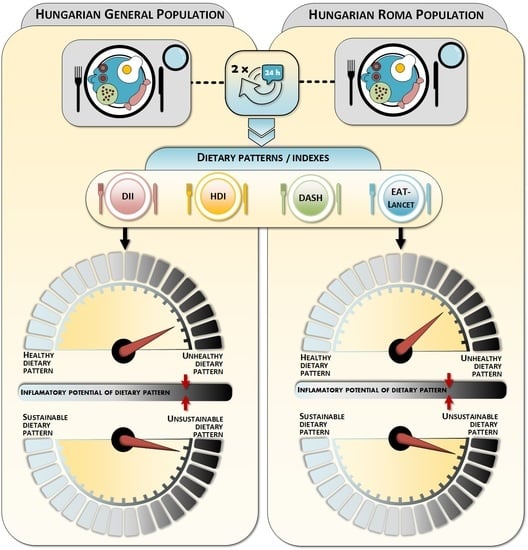
Deteriorated Dietary Patterns with Regards to Health and Environmental Sustainability among Hungarian Roma Are Not Differentiated from Those of the General Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Patterns Indexes
2.3. Data Analyses
2.4. Research Ethics
3. Results
Dietary Pattern Scores and Quality
4. Discussion
Limitations, Strengths and Future Outlooks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, V.S.; Kühlbrandt, C.; McKee, M. An examination of unmet health needs as perceived by Roma in Central and Eastern Europe. Eur. J. Public Health 2016, 26, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Ádány, R. Roma health is global ill health. Eur. J. Public Health 2014, 24, 702–703. [Google Scholar] [CrossRef] [Green Version]
- Cook, B.; Wayne, G.F.; Valentine, A.; Lessios, A.; Yeh, E. Revisiting the evidence on health and health care disparities among the Roma: A systematic review 2003–2012. Int. J. Public Health 2013, 58, 885–911. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Price, L.S.; Nattinger, A.B.; Rivera, F.; Hanson, R.; Gmehlin, C.G.; Perez, A.; Singh, S.; Buchan, B.W.; Ledeboer, N.A.; Pezzin, L.E. Racial Disparities in Incidence and Outcomes Among Patients With COVID-19. JAMA Netw. Open 2020, 3, e2021892. [Google Scholar] [CrossRef] [PubMed]
- Kabarriti, R.; Brodin, N.P.; Maron, M.I.; Guha, C.; Kalnicki, S.; Garg, M.K.; Racine, A.D. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Netw. Open 2020, 3, e2019795. [Google Scholar] [CrossRef]
- Khazanchi, R.; Evans, C.T.; Marcelin, J.R. Racism, Not Race, Drives Inequity Across the COVID-19 Continuum. JAMA Netw. Open 2020, 3, e2019933. [Google Scholar] [CrossRef] [PubMed]
- Chowkwanyun, M.; Reed, A.L. Racial Health Disparities and Covid-19—Caution and Context. N. Engl. J. Med. 2020, 383, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell, C.-R.C. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- EpiCentro. Report sulle Caratteristiche dei Pazienti Deceduti Positivia COVID-19 in Itali aIl Presente Report è Basato sui Dati Aggiornati al 17 Marzo 2020. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_17_marzo-v2.pdf (accessed on 11 January 2020).
- Ryan, D.H.; Ravussin, E.; Heymsfield, S. COVID 19 and the Patient with Obesity—The Editors Speak Out. Obesity 2020, 28, 847. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Dubowitz, T.; Heron, M.; Bird, C.E.; Lurie, N.; Finch, B.K.; Basurto-Dávila, R.; Hale, L.; Escarce, J.J. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am. J. Clin. Nutr. 2008, 87, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Kant, A.K.; Graubard, B.I.; Kumanyika, S.K. Trends in black-white differentials in dietary intakes of U.S. adults, 1971-2002. Am. J. Prev. Med. 2007, 32, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Satia, J.A. Diet-related disparities: Understanding the problem and accelerating solutions. J. Am. Diet. Assoc. 2009, 109, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.J.; Barrientos-Gutiérrez, T.; Corvalan, C.; Hyder, A.A.; Lazo-Porras, M.; Oni, T.; Wells, J.C.K. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 2019, 25, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Estimates and Official Numbers of Roma in Europe. Available online: https://rm.coe.int/CoERMPublicCommonSearchServices/DisplayDCTMContent?documentId=0900001680088ea9 (accessed on 29 June 2020).
- Brüggemann, C.; Friedman, E. The Decade of Roma Inclusion: Origins, Actors, and Legacies. Eur. Educ. 2017, 49, 1–9. [Google Scholar] [CrossRef]
- Sándor, J.; Kósa, Z.; Boruzs, K.; Boros, J.; Tokaji, I.; McKee, M.; Ádány, R. The decade of Roma Inclusion: Did it make a difference to health and use of health care services? Int. J. Public Health 2017, 62, 803–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valachovicova, M.; Krajcovicova-Kudlackova, M.; Ginter, E.; Paukova, V. Antioxidant vitamins levels--nutrition and smoking. Bratisl. Med. J. 2003, 104, 411–414. [Google Scholar]
- Davidová, E. Zp ůsob života a kultura: Změny ve hmotné kultuře Rom ů—Bydlení, strava. In Černobílý Život; Černá, M., Ed.; Gallery: Prague, Czech Republic, 2000; pp. 80–89. [Google Scholar]
- Stávková, J.; Brázdová, D.Z. Konzumace ovoce a zeleniny a jiné stravovací zvyklosti Romské populace. Hygiena 2014, 59, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Olišarová, V.; Tóthová, V.; Bártlová, S.; Dolák, F.; Kajanová, A.; Nováková, D.; Prokešová, R.; Šedová, L. Cultural Features Influencing Eating, Overweight, and Obesity in the Roma People of South Bohemia. Nutrients 2018, 10, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijova, E.; Madarasova Geckova, A.; Babinska, I. Do Eating Habits of the Population Living in Roma Settlements Differ from Those of the Majority Population in Slovakia? Cent. Eur. J. Public Health 2014, 22, S65–S68. [Google Scholar] [CrossRef] [Green Version]
- Fundación Secretariado Gitano. Health and the Roma Community: Analysis of the Situation in Europe. Available online: https://www.gitanos.org/upload/07/81/memoria_gral_fin.pdf (accessed on 29 June 2020).
- Ostrihoňová, T.; Bérešová, J. Occurrence of metabolic syndrome and its risk factors amongst a selected group of Roma inhabitants. Hygiena 2010, 55, 7–14. [Google Scholar]
- Hoxha, A.; Dervishi, G.; Bici, E.; Naum, A.; Seferi, J.; Risilia, K.; Tresa, E. Assessment of nutritional status and dietary patterns of the adult Roma community in Albania. Alban Med. J. 2013, 3, 32–38. [Google Scholar]
- Ciaian, P.; Cupák, A.; Pokrivčák, J.; Rizov, M. Food consumption and diet quality choices of Roma in Romania: A counterfactual analysis. Food Sec. 2018, 10, 437–456. [Google Scholar] [CrossRef] [Green Version]
- Bartosovic, I.; Hegyi, L.; Krcméry, V.; Hanobik, F.; Vasilj, V.; Rothova, P. Poverty & poor eating habits are two of the essential factors that affect the health condition of marginalized Roma population. CSW 2014, 15. [Google Scholar] [CrossRef]
- Sedova, L.; Tothova, V.; Novakova, D.; Olisarova, V.; Bartlova, S.; Dolak, F.; Kajanova, A.; Prokesova, R.; Adamkova, V. Qualification of Food Intake by the Roma Population in the Region of South Bohemia. Int. J. Environ. Res. Public Health 2018, 15, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirección General de Salud Pública Calidad e Innovación. Segunda Encuesta Nacional de Salud a Población Gitana. 2014. Available online: https://www.mscbs.gob.es/en/profesionales/saludPublica/prevPromocion/promocion/desigualdadSalud/docs/ENS2014PG.pdf (accessed on 29 June 2020).
- Lakatos, S.; Angyal, M.; Solymosy, J.; Bonifácz, S.; Kármán, J.; Csépe, P.; ForraiI, J.; Lökkös, A. Egyenlőség, Egészség és Roma/Cigány Közösség. Available online: http://ec.europa.eu/health/ph_projects/2004/action3/docs/2004_3_01_manuals_hu.pdf (accessed on 26 June 2020).
- Diószegi, J.; Pikó, P.; Kósa, Z.; Sándor, J.; Llanaj, E.; Ádány, R. Taste and Food Preferences of the Hungarian Roma Population. Front. Public Health 2020, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Balázs, P.; Rákóczi, I.; Fogarasi-Grenczer, A.; Foley, K.L. Birth-weight differences of Roma and non-Roma neonates–public health implications from a population-based study in Hungary. Cent. Eur. J. Public Health 2014, 22, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, M.V.; González, M.M.; San Fabián, J.L. La Situación de la Infancia Gitana en Asturias. Consejería de Bienestar Social y Vivienda; Gobierno del Principado de Asturias, Ed.; Instituto Asturiano de Atención Social a la Infancia, Familias y Adolescencia para el Observatorio de la Infancia y la Adolescencia del Principado de Asturias: Oviedo, Spain, 2011; p. 398. [Google Scholar]
- Observatorio de Salud Pública de Cantabria. Estudio Sobre Determinantes de la Salud de la Población Gitana Cántabra. Available online: https://www.ospc.es/ficheros/esp/ProyectosFicheros/9F9A2FBE-7DAC-6F48-1AC6-45C6340346CB.pdf/ (accessed on 29 June 2020).
- Pérez, F.J.; Arias-Gundín, O. La alimentación en un centro educativo donde las minorías son mayoría. Int. J. Behav. Dev. 2009, 2, 181–189. [Google Scholar]
- Velcheva, M.B. Research on the eating habits of pregnant Romani women and mothers of newborns in Bulgaria in 2015: Hristina Velcheva. Eur. J. Public Health 2016, 26, ckw174.015. [Google Scholar] [CrossRef] [Green Version]
- Szabóné Kármán, J. (Ed.) A magyarországi cigány/roma népesség kultúrantropológiai és orvosantropológiai megközelítésben. In Romológiai Füzetek; Debreceni Református Hittudományi Egyetem: Debrecen, Hungary, 2018; ISBN 978-615-5853-03-6. ISSN 2560-2209. [Google Scholar]
- Llanaj, E.; Vincze, F.; Kósa, Z.; Sándor, J.; Diószegi, J.; Ádány, R. Dietary Profile and Nutritional Status of the Roma Population Living in Segregated Colonies in Northeast Hungary. Nutrients 2020, 12, 2836. [Google Scholar] [CrossRef] [PubMed]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Ádány, R.; Pikó, P.; Fiatal, S.; Kósa, Z.; Sándor, J.; Bíró, É.; Kósa, K.; Paragh, G.; Bácsné Bába, É.; Veres-Balajti, I. Prevalence of Insulin Resistance in the Hungarian General and Roma Populations as Defined by Using Data Generated in a Complex Health (Interview and Examination) Survey. Int. J. Environ. Res. Public Health 2020, 17, 4833. [Google Scholar] [CrossRef] [PubMed]
- Llanaj, E.; Ádány, R.; Lachat, C.; D’Haese, M. Examining food intake and eating out of home patterns among university students. PLoS ONE 2018, 13, e0197874. [Google Scholar] [CrossRef] [Green Version]
- Llanaj, E.; Hanley-Cook, G.T. Adherence to Healthy and Sustainable Diets Is Not Differentiated by Cost, But Rather Source of Foods among Young Adults in Albania. Br. J. Nutr. 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Healthy Diet: Key Facts (29 April 2020 Update). Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 22 December 2020).
- Huijbregts, P.; Feskens, E.; Räsänen, L.; Fidanza, F.; Nissinen, A.; Menotti, A.; Kromhout, D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and the Netherlands: Longitudinal cohort study. BMJ 1997, 315, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating Dietary Habits Among Adults With Hypertension: DASH Dietary Accordance, NHANES 1988-1994 and 1999-2004. JAMA Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sándor, J.; Kósa, K.; Papp, M.; Fürjes, G.; Kőrösi, L.; Jakovljevic, M.; Ádány, R. Capitation-Based Financing Hampers the Provision of Preventive Services in Primary Health Care. Front. Public Health 2016, 4, 200. [Google Scholar] [CrossRef] [Green Version]
- Kósa, K.; Sándor, J.; Dobos, É.; Papp, M.; Fürjes, G.; Ádány, R. Human resources development for the operation of general practitioners’ cluster. Eur. J. Public Health 2013, 23, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Sándor, J.; Nagy, A.; Jenei, T.; Földvári, A.; Szabó, E.; Csenteri, O.; Vincze, F.; Sipos, V.; Kovács, N.; Pálinkás, A.; et al. Influence of patient characteristics on preventive service delivery and general practitioners’ preventive performance indicators: A study in patients with hypertension or diabetes mellitus from Hungary. Eur. J. Gen. Pract. 2018, 24, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rurik, I.; Ruzsinkó, K.; Jancsó, Z.; Antal, M. Nutritional Counseling for Diabetic Patients: A Pilot Study in Hungarian Primary Care. Ann. Nutr. Metab. 2010, 57, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Sándor, J.; Kósa, K.; Fürjes, G.; Papp, M.; Csordás, Á.; Rurik, I.; Ádány, R. Public health services provided in the framework of general practitioners’ clusters. Eur. J. Public Health 2013, 23, 530–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Mozaffarian, D.; Sy, S.; Huang, Y.; Liu, J.; Wilde, P.E.; Abrahams-Gessel, S.; Jardim, T.S.V.; Gaziano, T.A.; Micha, R. Cost-effectiveness of financial incentives for improving diet and health through Medicare and Medicaid: A microsimulation study. PLoS Med. 2019, 16, e1002761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gódor, A. The major trends of food consumption in Hungary. Deturope 2016, 8, 202–211. [Google Scholar]
- Kiss, A.; Pfeiffer, L.; Popp, J.; Oláh, J.; Lakner, Z. A Blind Man Leads a Blind Man? Personalised Nutrition-Related Attitudes, Knowledge and Behaviours of Fitness Trainers in Hungary. Nutrients 2020, 12, 663. [Google Scholar] [CrossRef] [Green Version]
- Stacey, D.; Hopkins, M.; Adamo, K.B.; Shorr, R.; Prud’homme, D. Knowledge translation to fitness trainers: A systematic review. Implement. Sci. 2010, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Healthy Lifestyles and Healthy Nutrition. Available online: https://eacea.ec.europa.eu/national-policies/en/content/youthwiki/74-healthy-lifestyles-and-healthy-nutrition-hungary (accessed on 23 November 2020).
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- 2020 Global Nutrition Report: Action on Equity to End Malnutrition. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report/ (accessed on 11 January 2021).
- Béné, C.; Prager, S.D.; Achicanoy, H.A.E.; Toro, P.A.; Lamotte, L.; Bonilla, C.; Mapes, B.R. Global map and indicators of food system sustainability. Sci. Data 2019, 6, 279. [Google Scholar] [CrossRef] [Green Version]
- Országos Egészségfejlesztési Intézet. Dietary Guidelines for the Adult Population in Hungary (Hungarian: Táplálkozási Ajánlások a Magyarországi Felnőtt Lakosság Számára). Available online: http://www.fao.org/3/a-as684o.pdf (accessed on 3 November 2020).
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat Intake and Mortality: A Prospective Study of Over Half a Million People. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef]
- Bellavia, A.; Stilling, F.; Wolk, A. High red meat intake and all-cause cardiovascular and cancer mortality: Is the risk modified by fruit and vegetable intake? Am. J. Clin. Nutr. 2016, 104, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Bodirsky, B.L.; Dietrich, J.P.; Martinelli, E.; Stenstad, A.; Pradhan, P.; Gabrysch, S.; Mishra, A.; Weindl, I.; Le Mouël, C.; Rolinski, S.; et al. The ongoing nutrition transition thwarts long-term targets for food security, public health and environmental protection. Sci. Rep. 2020, 10, 19778. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100, 476S–482S. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Strengthening Nutrition Action: A Resource Guide for Countries Based on the Policy Recommendations of the Second International Conference on Nutrition; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2019. [Google Scholar]
- Couch, S.C.; Saelens, B.E.; Khoury, P.R.; Dart, K.B.; Hinn, K.; Mitsnefes, M.M.; Daniels, S.R.; Urbina, E.M. Dietary Approaches to Stop Hypertension Dietary Intervention Improves Blood Pressure and Vascular Health in Youth With Elevated Blood Pressure. Hypertension 2020. [Google Scholar] [CrossRef]
- Ali Mohsenpour, M.; Fallah-Moshkani, R.; Ghiasvand, R.; Khosravi-Boroujeni, H.; Mehdi Ahmadi, S.; Brauer, P.; Salehi-Abargouei, A. Adherence to Dietary Approaches to Stop Hypertension (DASH)-Style Diet and the Risk of Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. J. Am. Coll. Nutr. 2019, 38, 513–525. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Yang, Z.; Duan, M.-L. Dietary approach to stop hypertension diet and risk of coronary artery disease: A meta-analysis of prospective cohort studies. Int. J. Food Sci. Nutr. 2019, 70, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, P.; Scarborough, P.; Lloyd, T.; Mizdrak, A.; Luben, R.; Mulligan, A.A.; Wareham, N.J.; Woodcock, J. Greater accordance with the Dietary Approaches to Stop Hypertension dietary pattern is associated with lower diet-related greenhouse gas production but higher dietary costs in the United Kingdom. Am. J. Clin. Nutr. 2015, 102, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Stinson, E.J.; Piaggi, P.; Votruba, S.B.; Venti, C.; Lovato-Morales, B.; Engel, S.; Krakoff, J.; Gluck, M.E. Is Dietary Nonadherence Unique to Obesity and Weight Loss? Results From a Randomized Clinical Trial. Obesity 2020, 28, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Branca, F.; Demaio, A.; Udomkesmalee, E.; Baker, P.; Aguayo, V.M.; Barquera, S.; Dain, K.; Keir, L.; Lartey, A.; Mugambi, G.; et al. A new nutrition manifesto for a new nutrition reality. Lancet 2020, 395, 8–10. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2019. [Google Scholar] [CrossRef]
- Nugent, R.; Levin, C.; Hale, J.; Hutchinson, B. Economic effects of the double burden of malnutrition. Lancet 2020, 395, 156–164. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2019, 395, 75–88. [Google Scholar] [CrossRef]
- Hawkes, C.; Ruel, M.T.; Salm, L.; Sinclair, B.; Branca, F. Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet 2020, 395, 142–155. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Kósa, Z.; Moravcsik-Kornyicki, Á.; Diószegi, J.; Roberts, B.; Szabó, Z.; Sándor, J.; Ádány, R. Prevalence of metabolic syndrome among Roma: A comparative health examination survey in Hungary. Eur. J. Public Health 2014, 25, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macejova, Z.; Kristian, P.; Janicko, M.; Halanova, M.; Drazilova, S.; Antolova, D.; Marekova, M.; Pella, D.; Madarasova-Geckova, A.; Jarcuska, P. The Roma Population Living in Segregated Settlements in Eastern Slovakia Has a Higher Prevalence of Metabolic Syndrome, Kidney Disease, Viral Hepatitis B and E, and Some Parasitic Diseases Compared to the Majority Population. Int. J. Environ. Res. Public Health 2020, 17, 3112. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Variable † | Hungarian General (n = 344) | Hungarian Roma (n = 359) | p‡ | |
|---|---|---|---|---|---|
| Basic characteristics | Demographics | Age (years)—mean (std. dev.) | 44 (12) | 43 (12) | NS |
| Females—n (%) | 188 (52.4) | 248 (72.1) | *** | ||
| Education | Elementary—n (%) | 76 (21.2) | 292 (84.9) | *** | |
| Secondary/Vocational education—n (%) | 230 (64.1) | 52 (15.1) | |||
| University degree or higher—n (%) | 53 (14.8) | 0 (0) | |||
| Economic activity | Some type of full-/part-time employment—n (%) | 296 (82.5) | 256 (74.4) | ** | |
| Student—n (%) | 8 (2.2) | 0 (0) | |||
| Unable to work/Retired—n (%) | 40 (11.1) | 32 (9.3) | |||
| Unemployed—n (%) | 15 (4.2) | 56 (16.3) | |||
| Marital status | Unmarried/Divorced/Widowed—n (%) | 136 (37.9) | 113 (32.8) | NS | |
| Married—n (%) | 223 (62.1) | 231 (67.2) | |||
| Perceived financial wellbeing | Good or very good—n (%) | 115 (32.8) | 51 (15.0) | *** | |
| Fair—n (%) | 190 (54.1) | 186 (54.7) | |||
| Challenging or very challenging—n (%) | 46 (13.1) | 103 (30.3) | |||
| Nutritional characteristics | Energy intake | Energy (kJ/day) | 9146.9 (8824.7; 9469.1) | 8836.9 (8537.0; 9136.8) | NS |
| Energy (kcal/day) | 2188.3 (2111.2; 2265.3) | 2114.1 (2042.4; 2185.8) | NS | ||
| Anthropometrics | BMI (kg/m2) | 27.26 (26.7; 27.8) | 27.66 (27.0; 28.4) | NS | |
| Macronutrient intake | Fiber (%E) | 3.9 (3.7; 4.0) | 4.0 (3.8; 4.2) | NS | |
| Fiber (g/1000 kcal) | 9.7 (9.2; 10.1) | 9.9 (9.4; 10.4) | NS | ||
| Protein (%E) | 15.6 (15.2; 15.9) | 15.1 (14.7; 15.4) | * | ||
| Fat (%E) | 37.2 (36.3; 38.0) | 36.1 (35.2; 37.0) | NS | ||
| SFA (%E) | 10.7 (10.3; 11.1) | 10.66 (10.3; 11.0) | NS | ||
| Carbohydrates (%E) | 46.2 (45.3; 47.1) | 48.2 (47.2; 49.2) | ** | ||
| Sugar (g) | 96.3 (89.0; 103.5) | 101.5 (94.1; 108.8) | NS | ||
| Sugar (%E) | 17.0 (16.0; 18.0) | 18.8(17.7; 19.8) | * | ||
| UFA (g) | 20.9 (20.4; 21.4) | 19.7 (19.1; 20.2) | ** | ||
| Cholesterol (mg/1000 kcal) | 172.9 (164.7; 181.0) | 159.5 (152.2; 166.8) | * | ||
| Micronutrient intake | Magnesium (mg/1000 kcal) | 193.1 (166.0; 220.2) | 181.6 (173.8; 189.5) | NS | |
| Calcium (mg/1000 kcal) | 248.5 (233.51; 263.49) | 248.0 (234.4; 261.7) | NS | ||
| Potassium (mg/1000 kcal) | 1391.1 (1311.4; 1470.8) | 1435.3 (1353.3; 1517.4) | NS | ||
| Sodium (mg/1000 kcal) | 2628.1 (2522.8; 2733.4) | 2458.3 (2365.7; 2550.8) | * | ||
| Dietary Indicator | Hungarian General | Hungarian Roma | |||||
|---|---|---|---|---|---|---|---|
| Both Sexes (A) | Females (C) | Males (E) | Both Sexes (B) | Females (D) | Males (F) | ||
| HDI | Very low | 27 (7.5) | 13 (6.9) | 14 (8.2) | 23 (6.7) | 18 (7.3) | 5 (5.2) |
| Low | 172 (47.9) | 91 (48.6) | 81 (47.4) | 152 (44.2) | 113 (45.6) | 39 (40.6) | |
| Moderate | 140 (39.0) | 75 (39.9) | 65 (37.0) | 158 (45.9) | 111 (44.7) | 47 (48.9) | |
| High | 20 (5.6) | 9 (4.6) | 11 (6.4) | 11 (3.2) | 6 (2.4) | 5 (5.2) | |
| DASH | Non-accordant | 341 (95.0) | 177 (94.1) | 164 (95.9) | 330 (95.9) | 240 (96.8) | 90 (93.8) |
| Accordant | 18 (5.0) | 11 (5.9) | 7 (4.1) | 14 (4.1) | 8 (3.2) | 6 (6.2) | |
| NB-EAT | Low | 324 (90.3) | 171 (91.0) | 153 (89.5) | 305 (88.7) | 223 (89.9) | 82 (85.4) |
| Moderate | 35 (9.7) | 17 (9.0) | 18 (10.5) | 39 (11.3) | 25 (10.1) | 14 (14.6) | |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| DII | Tertile 1 | 119 (33.1) | 66 (35.1) | 53 (31.0) | 115 (33.5) | 84 (33.9) | 31 (32.3) |
| Tertile 2 | 109 (30.4) | 50 (26.6) | 59 (34.5) | 126 (36.6) | 90 (36.3) | 36 (37.5) | |
| Tertile 3 | 131 (36.5) | 72 (38.3) | 59 (34.5) | 103 (29.9) | 74 (29.8) | 29 (30.2) | |
| DASH score (0–9): median (IQR) | 1.5 (1.5; 2.0) | 1.5 (1.5; 2.0) | 1.5 (1.5; 2.0) | 1.5 (1.5; 2.0) | 1.5 (1.5; 2.0) | 1.5 (1.5; 2.0) | |
| NB-EAT score (0–12): median (IQR) | 2.0 (2.0; 3.0) | 2.0 (2.0; 3.0) | 2.0 (2.0; 3.0) | 2.0 (2.0; 3.0) | 2.0 (2.0; 3.0) | 3.0 (3.0; 4.0) | |
| HDI score (0–7): median (IQR) | 3.0 (3.0; 4.0) | 3.0 (3.0; 4.0) | 3.0 (3.0; 4.0) | 3.0 (3.0; 4.0) | 3.0 (3.0; 4.0) | 4.0 (4.0; 5.0) | |
| DII score (−4.60–3.12): median (IQR) | −1.26 (−1.49; −1.06) | −1.31 (−1.80; −0.92) | −1.26 (−1.45; −1.02) | −1.40 (−1.65; −1.23) | −1.34 (−1.64; −1.12) | −1.59 (−1.92; −1.27) | |
| Dietary Score | MODEL 1 (β [95%CI]) | MODEL 2 (β [95%CI]) | MODEL 3 (β [95%CI]) |
|---|---|---|---|
| DASH † | −0.023 [−0.176; 0.129] | −0.084 [−0.286; 0.117] | −0.049 [−0.254; 0.156] |
| HDI † | 0.038 [−0.131; 0.207] | −0.003 [−0.229; 0.223] | −0.001 [−0.231; 0.230] |
| DII ‡ | −0.147 [−0.344; 0.049] | −0.450 [−0.709; −0.191] | −0.455 [−0.720; −0.191] |
| NB-EAT † | 0.021 [−0.073; 0.114] | −0.024 [−0.183; 0.136] | −0.017 [−0.179; 0.144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanaj, E.; Vincze, F.; Kósa, Z.; Bárdos, H.; Diószegi, J.; Sándor, J.; Ádány, R. Deteriorated Dietary Patterns with Regards to Health and Environmental Sustainability among Hungarian Roma Are Not Differentiated from Those of the General Population. Nutrients 2021, 13, 721. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030721
Llanaj E, Vincze F, Kósa Z, Bárdos H, Diószegi J, Sándor J, Ádány R. Deteriorated Dietary Patterns with Regards to Health and Environmental Sustainability among Hungarian Roma Are Not Differentiated from Those of the General Population. Nutrients. 2021; 13(3):721. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030721
Chicago/Turabian StyleLlanaj, Erand, Ferenc Vincze, Zsigmond Kósa, Helga Bárdos, Judit Diószegi, János Sándor, and Róza Ádány. 2021. "Deteriorated Dietary Patterns with Regards to Health and Environmental Sustainability among Hungarian Roma Are Not Differentiated from Those of the General Population" Nutrients 13, no. 3: 721. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030721