Effect of a Food for Special Medical Purposes for Muscle Recovery, Consisting of Arginine, Glutamine and Beta-Hydroxy-Beta-Methylbutyrate on Body Composition and Skin Health in Overweight and Obese Class I Sedentary Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
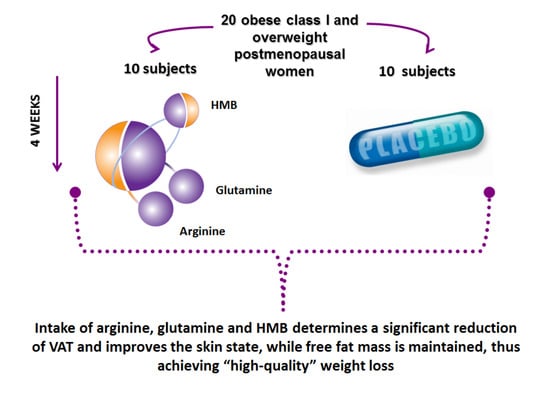
2.1. Study Disegn
2.2. Study Population
2.3. Dietary Supplement Intervention
2.4. Adverse Events
2.5. Biochemical Markers
2.6. Anthropometric Measurements and Dietary Advice
2.7. Body Composition
2.8. Assessment of Skin Condition
2.9. Primary and Secondary Endpoints
2.10. Sample Size
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | amino acids |
| BMI | Body Mass Index |
| CI | confidence interval |
| eGFR | Glomerular Filtration Rate |
| DS | dietary supplements |
| DXA | dual-energy X-ray absorptiometry |
| FBG | Fasting blood glucose |
| FM | fat mass |
| FFM | fat free mass |
| FSMP | food for special medical purposes |
| HDL-C | high-density lipoprotein-cholesterol |
| HMB | beta-hydroxy-beta-methylbutyrate |
| LDL-C | low-density lipoprotein-cholesterol |
| TC | total cholesterol |
| TG | triglycerides |
| SD | standard deviation |
| VAT | visceral adipose tissue. |
References
- World Health Organization Obesity. Preventing and managing the global epidemic. WHO Tech. Rep. Ser. 2020, 894, 252. [Google Scholar]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef]
- Acosta, A.; Dayyeh, B.K.A.; Port, J.D.; Camilleri, M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut 2014, 63, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.S.; Cram, A.E.; Chao, M.; Pang, J.; McKeon, M. Belt Lipectomy for Circumferential Truncal Excess: The University of Iowa Experience. Plast. Reconstr. Surg. 2003, 111, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Hector, A.J.; Phillips, S.M. Considerations for protein intake in managing weight loss in athletes. Eur. J. Sport Sci. 2015, 15, 21–28. [Google Scholar] [CrossRef]
- Cocate, P.G.; Natali, A.J.; de Oliveira, A.; Alfenas, R.C.; Hermsdorff, H.H.M. Consumption of branched-chain amino acids is inversely associated with central obesity and cardiometabolic features in a population of Brazilian middle-aged men: Potential role of leucine intake. J. Nutr. Health Aging 2015, 19, 771–777. [Google Scholar] [CrossRef]
- van den Broek, M.; de Heide, L.J.M.; Emous, M.; Wijma, R.B.; Veeger, N.J.G.M.; Wolthuis, A.; Laskewitz, A.J.; Heiner-Fokkema, M.R.; Muller Kobold, A.C.; Wolffenbuttel, B.H.R.; et al. Satiety and gastrointestinal hormones during a Mixed Meal Tolerance Test after gastric bypass surgery: Association with plasma amino acid concentrations. Surg. Obes. Relat. Dis. 2018, 14, 1106–1117. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef]
- Kujawska-Luczak, M.; Suliburska, J.; Markuszewski, L.; Pupek-Musialik, D.; Jabecka, A.; Bogdañski, P. The effect of L-arginine and ascorbic acid on the visceral fat and the concentrations of metalloproteinases 2 and 9 in high-fat-diet rats. Endokrynol. Pol. 2015, 66, 526–532. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Grabanska, K.; Musialik, K.; Cieslewicz, A.; Skoluda, A.; Jablecka, A. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 816–823. [Google Scholar]
- Zajac, A.; Poprzȩcki, S.; Zebrowska, A.; Chalimoniuk, M.; Langfort, J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. J. Strength Cond. Res. 2010, 24, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligthart-Melis, G.C.; Van De Poll, M.C.G.; Boelens, P.G.; Dejong, C.H.C.; Deutz, N.E.P.; Van Leeuwen, P.A.M. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am. J. Clin. Nutr. 2008, 87, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, C.; Gannon, J.; Allan Walker, W. Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 2002, 75, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Albert, F.J.; Morente-Sánchez, J.; Ortega, F.B.; Castillo, M.J.; Gutiérrez, Á. Eficacia de la suplementación con β-hydroxy-β-methylbutyrate (hmb) en el deporte: Actualización e implicación práctica. Nutr. Hosp. 2015, 32, 20–33. [Google Scholar]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: A randomized, double-blind pilot trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Olveira, G.; Olveira, C.; Doña, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martínez, E.; Rojo-Martínez, G.; Martín-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2016, 35, 1015–1022. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein recommendations for weight loss in elite athletes: A focus on body composition and performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Stout, J.R.; Fukuda, D.H.; Kendall, K.L.; Smith-Ryan, A.E.; Moon, J.R.; Hoffman, J.R. β-Hydroxy-β-methylbutyrate (HMB) supplementation and resistance exercise significantly reduce abdominal adiposity in healthy elderly men. Exp. Gerontol. 2015, 64, 33–34. [Google Scholar] [CrossRef]
- Hashempour, A.; Hooshmand, S.; Tabesh, M.R.; Alizadeh, Z. Effect of 6-week HMB (beta-hydroxy-beta methylbutyrate) supplementation on muscle strength and body composition in sedentary overweight women. Obes. Med. 2019, 15, 100115. [Google Scholar] [CrossRef]
- Takaoka, M.; Okumura, S.; Seki, T.; Ohtani, M. Effect of amino-acid intake on physical conditions and skin state: A randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Biochem. Nutr. 2019, 65, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.L.; Groth, A.K.; Branco, A.B. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 281–288. [Google Scholar] [CrossRef]
- Peng, X.; Yan, H.; You, Z.; Wang, P.; Wang, S. Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients. Burns 2005, 31, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.N.; Winter, D.C.; Bouchier-Hayes, D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen. 2006, 14, 376–386. [Google Scholar] [CrossRef]
- Kirk, S.J.; Hurson, M.; Regan, M.C.; Holt, D.R.; Wasserkrug, H.L.; Barbul, A.; Daly, J.M.; Flye, M.W. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993, 114, 155–160. [Google Scholar]
- Williams, J.Z.; Abumrad, N.; Barbul, A. Effect of a specialized amino acid mixture on human collagen deposition. Ann. Surg. 2002, 236, 369–375. [Google Scholar] [CrossRef]
- Kapoor, E.; Collazo-Clavell, M.L.; Faubion, S.S. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo Clin. Proc. 2017, 92, 1552–1558. [Google Scholar] [CrossRef]
- Milewicz, A.; Tworowska, U.; Demissie, M. Menopausal obesity—Myth or fact? Climacteric 2001, 4, 273–283. [Google Scholar]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Aminian, S.; et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Haffner, S.M.; Kennedy, E.; Gonzalez, C.; Stern, M.P.; Miettinen, H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996, 19, 1138–1141. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Sakai, S.; Yoshida, H.; Okado, N.; Kaneda, H.; Masuda, Y.; Urushibata, O. Oral hyaluronan relieves wrinkles: A double-blinded, placebo-controlled study over a 12-week period. Clin. Cosmet. Investig. Dermatol. 2017, 10, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, J.A. The Consolidated Standards of Reporting Trials (CONSORT): Guidelines for reporting randomized trials. Nurs. Res. 2005, 54, 128–132. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas 2020, 141, 33–38. [Google Scholar] [CrossRef]
- Townsend, J.R.; Hoffman, J.R.; Gonzalez, A.M.; Jajtner, A.R.; Boone, C.H.; Robinson, E.H.; Mangine, G.T.; Wells, A.J.; Fragala, M.S.; Fukuda, D.H.; et al. Effects of β-hydroxy-β-methylbutyrate free acid ingestion and resistance exercise on the acute endocrine response. Int. J. Endocrinol. 2015, 2015, 856708. [Google Scholar] [CrossRef] [Green Version]
- Yosipovitch, G.; DeVore, A.; Dawn, A. Obesity and the skin: Skin physiology and skin manifestations of obesity. J. Am. Acad. Dermatol. 2007, 56, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Orpheu, S.C.; Coltro, P.S.; Scopel, G.P.; Gomez, D.S.; Rodrigues, C.J.; Modolin, M.L.A.; Faintuch, J.; Gemperli, R.; Ferreira, M.C. Collagen and elastic content of abdominal skin after surgical weight loss. Obes. Surg. 2010, 20, 480–486. [Google Scholar] [CrossRef]
- Light, D.; Arvanitis, G.M.; Abramson, D.; Glasberg, S.B. Effect of weight loss after bariatric surgery on skin and the extracellular matrix. Plast. Reconstr. Surg. 2010, 125, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.; Marti, G.; Nastai, M.; Mallalieu, J.; Shermak, M.A. Biomechanical properties of skin in massive weight loss patients. Obes. Surg. 2010, 20, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Sami, K.; Elshahat, A.; Moussa, M.; Abbas, A.; Mahmoud, A. Image analyzer study of the skin in patients with morbid obesity and massive weight loss. Eplasty 2015, 15, e4. [Google Scholar] [PubMed]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr. Metab. 2008, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]



| Outcome | Intervention Group (n = 10) Mean ± SD | Placebo Group (n = 10) Mean ± SD | Total Sample (n = 20) Mean ± SD | p-Value between Groups |
|---|---|---|---|---|
| Age (years) | 54.60 ± 5.64 | 54.20 ± 5.29 | 54.44 ± 5.33 | 0.872 |
| Total Cholesterol (mg/dL) | 201.70 ± 23.45 | 198.80 ± 40.07 | 200.25 ± 31.76 | 0.846 |
| LDL Cholesterol (mg/dL) | 119.70 ± 10.40 | 121.44 ± 34.07 | 120.57 ± 22.24 | 0.879 |
| HDL Cholesterol (mg/dL) | 66.90 ± 15.39 | 64.70 ± 12.50 | 65.80 ± 13.95 | 0.730 |
| Triglycerides (mg/dL) | 100.80 ± 28.07 | 99.60 ± 25.24 | 100.20 ± 26.66 | 0.921 |
| Glycemia (mg/dL) | 96.70 ± 8.47 | 96.00 ± 12.71 | 96.35 ± 10.59 | 0.886 |
| Insulin (mcIU/mL) | 11.77 ± 4.49 | 11.42 ± 4.11 | 11.60 ± 4.30 | 0.859 |
| HOMA index (pt) | 2.85 ± 1.25 | 2.79 ± 1.30 | 2.82 ± 1.28 | 0.922 |
| Aspartate Aminotransferase (UI/L) | 20.50 ± 4.17 | 19.10 ± 3.21 | 19.80 ± 3.69 | 0.411 |
| Alanine Aminotransferase (UI/L) | 22.70 ± 7.09 | 20.20 ± 5.18 | 21.45 ± 6.14 | 0.380 |
| Gamma Glutamyl Transferase (U/L) | 26.10 ± 7.50 | 23.00 ± 7.99 | 24.55 ± 7.75 | 0.383 |
| Creatinine (mg/dL) | 0.70 ± 0.15 | 0.73 ± 0.06 | 0.72 ± 0.11 | 0.515 |
| eGFR mL/min | 90.37 ± 15.07 | 88.90 ± 9.90 | 89.64 ± 12.49 | 0.800 |
| Height (m) | 1.60 ± 0.05 | 1.63 ± 0.05 | 1.62 ± 0.05 | 0.272 |
| Weight (kg) | 74.72 ± 6.54 | 78.69 ± 9.86 | 76.71 ± 8.20 | 0.303 |
| Body Mass Index (kg/m2) | 29.20 ± 2.78 | 29.82 ± 3.01 | 29.51 ± 2.90 | 0.635 |
| Waist circumference (cm) | 95.45 ± 5.96 | 99.00 ± 9.61 | 97.23 ± 7.79 | 0.334 |
| Visceral Adipose Tissue (g) | 984.30 ± 398.71 | 1119.20 ± 455.96 | 1051.75 ± 427.34 | 0.490 |
| Fat Mass (g) | 32,419.40 ± 5642.07 | 32,044.90 ± 6251.10 | 32,232.15 ± 5946.59 | 0.890 |
| Fat Free Mass (g) | 40,443.40 ± 2412.14 | 44,010.50 ± 7942.71 | 42,226.95 ± 5177.42 | 0.191 |
| Bright (pt) | 2.60 ± 0.97 | 2.60 ± 0.97 | 2.60 ± 0.97 | 1.000 |
| Elasticity (pt) | 2.70 ± 0.95 | 2.80 ± 0.63 | 2.75 ± 0.79 | 0.785 |
| Wrinkle (pt) | 2.90 ± 0.57 | 2.90 ± 0.74 | 2.90 ± 0.66 | 1.000 |
| Variable | Intervention Intra-Group β (95%CI) | Placebo Intra-Group β (95%CI) | Intervention Effect between Groups Mean Difference |
|---|---|---|---|
| Weight (kg) | −1.540 (−2.484; −0.596) | −0.370 (−1.314; 0.574) | −1.170 |
| Body Mass Index (kg/m2) | −0.597 (−0.977; −0.217) | −0.080 (−0.460; 0.300) | −0.517 |
| Waist circumference (cm) | −1.550 (−3.240; 0.140) | −0.050 (−1.740; 1.640) | −1.500 |
| Visceral Adipose Tissue (g) | −93.800 (−140.650; −46.950) | 59.800 (12.950; 106.650) | −153.600 |
| Fat Mass (g) | −1166.300 (−2028.800; −303.800) | 225.100 (−637.400; 1087.600) | −1391 |
| Fat Free Mass (g) | −230.500 (−915.600; 454.600) | −576.200 (−1261.300; 108.900) | 345.600 |
| Variable | Intervention Intra-Group β (95%CI) | Placebo Intra-Group β (95%CI) | Intervention Effect between Groups Mean difference |
|---|---|---|---|
| Bright | 1.400 (0.758; 2.042) | 0.000 (−0.313; 0.313) | 1.400 |
| Elasticity | 0.900 (0.239; 1.561) | 0.000 (−0.313; 0.313) | 0.900 |
| Wrinkles | 0.800 (0.276; 1.324) | −0.100 (−0.310; 0.110) | 0.900 |
| Total score | 3.000 (1.871; 4.129) | −0.100 (−0.477; 0.277) | 3.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Nichetti, M.; Peroni, G.; Naso, M.; Faliva, M.A.; Iannello, G.; Di Paolo, E.; Perna, S. Effect of a Food for Special Medical Purposes for Muscle Recovery, Consisting of Arginine, Glutamine and Beta-Hydroxy-Beta-Methylbutyrate on Body Composition and Skin Health in Overweight and Obese Class I Sedentary Postmenopausal Women. Nutrients 2021, 13, 975. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030975
Rondanelli M, Nichetti M, Peroni G, Naso M, Faliva MA, Iannello G, Di Paolo E, Perna S. Effect of a Food for Special Medical Purposes for Muscle Recovery, Consisting of Arginine, Glutamine and Beta-Hydroxy-Beta-Methylbutyrate on Body Composition and Skin Health in Overweight and Obese Class I Sedentary Postmenopausal Women. Nutrients. 2021; 13(3):975. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030975
Chicago/Turabian StyleRondanelli, Mariangela, Mara Nichetti, Gabriella Peroni, Maurizio Naso, Milena Anna Faliva, Giancarlo Iannello, Enrica Di Paolo, and Simone Perna. 2021. "Effect of a Food for Special Medical Purposes for Muscle Recovery, Consisting of Arginine, Glutamine and Beta-Hydroxy-Beta-Methylbutyrate on Body Composition and Skin Health in Overweight and Obese Class I Sedentary Postmenopausal Women" Nutrients 13, no. 3: 975. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030975