Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue
Abstract
:1. Introduction
2. Materials and Methods
3. Results
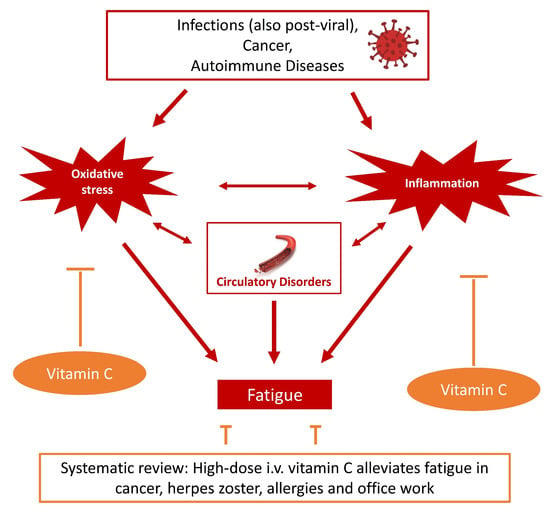
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. (Lausanne) 2020, 7, 606824. [Google Scholar] [CrossRef]
- Bleijenberg, G.; van der Meer, J.W.M. Chapter 442: Chronic Fatigue Syndrome. In Harrison’s Principles of Internal Medicine, 20e; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- NIHR. Living with Covid19. Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ (accessed on 25 February 2021).
- NIH. COVID-19 Treatment Guidlines: Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 15 March 2021).
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Schonrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Chiscano-Camon, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with Community Acquired Pneumonia Exhibit Depleted Vitamin C Status and Elevated Oxidative Stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, B.; Yin, L.; Guo, M.; Shi, H.; Zhu, Z.; Zhang, L.; He, J.; Ling, Y.; Gao, M.; et al. Vitamin C supplementation is necessary for patients with coronavirus disease: An ultra-high-performance liquid chromatography-tandem mass spectrometry finding. J. Pharm Biomed. Anal. 2021, 196, 113927. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. Pilot Study Antioxid. 2021, 10, 257. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczynski, P.; Zhitkovich, A.; Rubis, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: Concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Kuiper, C.; Vissers, M.C.; Hicks, K.O. Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue. Free Radic. Biol. Med. 2014, 77, 340–352. [Google Scholar] [CrossRef]
- Patterson, T.; Isales, C.M.; Fulzele, S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021, 12, 14–26. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharm. 2013, 72, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Mizuno, H.; Yanagisawa, A. High-dose intravenous vitamin C improves quality of life in cancer patients. Pers. Med. Universe 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med. Sci. Monit. 2012, 18, CR215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous vitamin C in the treatment of allergies: An interim subgroup analysis of a long-term observational study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of Intravenous High Dose Vitamin C on Postoperative Pain and Morphine Use after Laparoscopic Colectomy: A Randomized Controlled Trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef]
- Suh, S.Y.; Bae, W.K.; Ahn, H.Y.; Choi, S.E.; Jung, G.C.; Yeom, C.H. Intravenous Vitamin C Administration Reduces Fatigue in Office Workers: A Double-blind Randomized Controlled Trial. Nutr. J. 2012, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Vissers, M.C.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat Care 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef]
- Elera-Fitzcarrald, C.; Rocha, J.; Burgos, P.I.; Ugarte-Gil, M.F.; Petri, M.; Alarcon, G.S. Measures of Fatigue in Patients With Rheumatic Diseases: A Critical Review. Arthritis Care Res. 2020, 72 (Suppl. 10), 369–409. [Google Scholar] [CrossRef]
- Mohandas, H.; Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Rohini Thevi, G.V. Cancer-related fatigue treatment: An overview. J. Cancer Res. Ther. 2017, 13, 916–929. [Google Scholar] [CrossRef]
- Repka, C.P.; Hayward, R. Effects of an Exercise Intervention on Cancer-Related Fatigue and Its Relationship to Markers of Oxidative Stress. Integr. Cancer Ther. 2018, 17, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative Stress is a Convincing Contributor to Idiopathic Chronic Fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef]
- Morris, G.; Stubbs, B.; Kohler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med. Rev. 2018, 41, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.M.; Thomas, W.; Zhu, X.; Diebes, A.; McElvain, G.; Baechler, E.; Gross, M. Oxidative stress and fatigue in systemic lupus erythematosus. Lupus 2012, 21, 984–992. [Google Scholar] [CrossRef]
- Pearson, E.J.M.; Morris, M.E.; di Stefano, M.; McKinstry, C.E. Interventions for cancer-related fatigue: A scoping review. Eur. J. Cancer Care 2018, 27. [Google Scholar] [CrossRef]
- Korte, S.M.; Straub, R.H. Fatigue in inflammatory rheumatic disorders: Pathophysiological mechanisms. Rheumatology 2019, 58, v35–v50. [Google Scholar] [CrossRef]
- Lunec, J.; Blake, D.R. The determination of dehydroascorbic acid and ascorbic acid in the serum and synovial fluid of patients with rheumatoid arthritis (RA). Free Radic. Res. Commun. 1985, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Mehta, H.C.; Sood, A.K.; Kaur, J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin. Chim. Acta 2003, 338, 123–129. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy-Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: A role for NF-kappaB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Sturzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13. [Google Scholar] [CrossRef]
- Bhadelia, N.; Belkina, A.C.; Olson, A.; Winters, T.; Urick, P.; Lin, N.; Rifkin, I.; Kataria, Y.; Yuen, R.R.; Sagar, M.; et al. Distinct Autoimmune Antibody Signatures Between Hospitalized Acute COVID-19 Patients, SARS-CoV-2 Convalescent Individuals, and Unexposed Pre-Pandemic Controls. medRxiv 2021. [Google Scholar] [CrossRef]
- Rottoli, M.; La Gioia, S.; Frigeni, B.; Barcella, V. Pathophysiology, assessment and management of multiple sclerosis fatigue: An update. Expert Rev. Neurother. 2017, 17, 373–379. [Google Scholar] [CrossRef]
- Cramp, F. The role of non-pharmacological interventions in the management of rheumatoid-arthritis-related fatigue. Rheumatology 2019, 58, v22–v28. [Google Scholar] [CrossRef] [Green Version]
- Griggs, S.; Morris, N.S. Fatigue Among Adults With Type 1 Diabetes Mellitus and Implications for Self-Management: An Integrative Review. Diabetes Educ. 2018, 44, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Study Type; Number of Patients (n); Underlying Disease | IV Vitamin C Dose | Additional Interventions | Estimation of Fatigue | Impact on Fatigue and Related Parameters |
|---|---|---|---|---|---|
| Oncology | |||||
| [21] | Single-center, phase II, randomized clinical trial; n = 97; extensively pretreated patients with advanced, refractory non-small-cell lung cancer | 1 g/kg bw, 3 times/week, 25 treatments in total | Vitamin C group received concurrently modulated electro-hyperthermia; both groups received best supportive care | EORTC QLQ-C30 | Fatigue (mean ± SD) Verum group: pre: 46.48 ± 17.52, post: 20.63 ± 18.14 (* p < 0.0001) Control group: pre: 39.93 ± 20.59, post: 61.34 ± 25.32 (* p < 0.0001) (** p< 0.0001) Physical function ↑ (** p < 0.0001) Cognitive function (** p = 0.1026) Dyspnea ↓ (** p < 0.0001) Insomnia (** p = 0.0772 Pain ↓(p** p < 0.0001) |
| [22] | Single-center phase I clinical trial; n = 17; patients with refractory, advanced solid tumors (stage III-IV; colon, pancreas, breast, etc.) | 0.8–3 g/kg bw, 4 times/week for 4 weeks | None | EORTC QLQ-C30 | Fatigue ↓ (pre: 49/ post 11) Physical function ↑ (pre 69/post 87) Cognitive function ↑ (pre 75/post 83) Dyspnea ↓ (pre 24/post 0) Insomnia ↓ (pre 31/post 17) Pain ↓ (pre 36/ post 0) |
| [23] | Multi-center, prospective observational trial; n = 60; patients with advanced tumors (lung, breast, stomach, colonm etc.) | Increasing dosages up to 50 g and more to achieve plasma levels of 350–400 mg/dL 2 times/week for 4 weeks | +/− chemotherapy | EORTC QLQ-C30 | Fatigue (mean ± SD) Pre: 42.4 ± 28.7 post: 28.4 25.7 (* p < 0.01) Physical function ↑ (* p < 0.05) Cognitive function ↑ (* p < 0.01) Dyspnea (not significant) Insomnia ↓ (* p < 0.01) Pain ↓ (* p < 0.05) |
| [24] | Single-center, prospective before-and-after study; n = 39, terminal cancer patients (stomach, colon, lungs, breast, gall bladder, etc.) | 10 g 2 times/week for one week | None | EORTC QLQ-C30 | Fatigue (mean ± SD) Pre: 52 ± 24, post: 40 ± 19 (* p = 0.001) Physical function ↑ (* p = 0.037) Cognitive function ↑ (* p = 0.002) Dyspnea (p = 0.051) Insomnia ↓ (* p = 0.029) Pain ↓ (* p = 0.013) |
| [25] | Multi-center, retrospective, cohort study; n = 125, patients with breast cancer UICC IIa-IIIb | ≥7.5 g at least 1 time/week for at least 4 weeks | +/− chemotherapy, radiation | 3-point Likert scale | Fatigue (mean ± SD) During adjuvant therapy (first 6 months after operation): Verum: pre: 1.53 ± 1.11, post: 0.71 ± 0.89 Control: pre 1.68 ± 1.004, post: 1.24 ± 0.936 (** p = 0.004) During after care (6–12 month after operation): Verum: 0.34 ± 0.58 Control: 0.64 ± 0.718 (** p = 0.023) Sleep disorders ↓ (** p = 0.005) Depression ↓ (** p = 0.01) |
| Infection, allergies | |||||
| [26] | Multi-center, prospective observational trial; n = 67; patients with herpes zoster infection | 7.5 or 15 g; on average 8 infusions within 2–3 weeks | 55.8% received anti-infective drug | 4-point Likert scale | Fatigue improved in 78.2% of the patients; Impaired concentration improved in 81.8% of the patients |
| [27] | Multi-center, prospective observational trial; n = 71; patients with respiratory and cutaneous allergies | 7.5 g; 2–3 times/week for 2–3 weeks in acute and 11–12 weeks in chronic states | 35 % received anti-allergic drugs | 4-point Likert scale | Sum score (0–12) of the 4 symptoms: fatigue, sleep disorders, depression, and lack of mental concentration decreased from 5.93 to 1.09 (* p < 0.0001) Fatigue improved in 93.5% of patients Sleep disorders improved in 92.5%, depression in 95.5%, and impaired concentration in 91.7% |
| Others | |||||
| [28] | Single-center, randomized, double-blind, controlled clinical trial; n = 97; patients under-going laparoscopic colectomy | 50 mg/ kg bw; Single application after induction of anesthesia | Analgesics | NRS (0–10) | No significant differences in fatigue score 2, 6, and 24 h post operation Pain ↓ (** p < 0.05) |
| [29] | Multi-center, randomized, double-blind, controlled clinical trial; n = 147; apparently healthy full-time worker | 10 g, single application | None | NRS (0–10) | Fatigue (mean ± SD) Verum: Pre: 5.64 ± 2.02, after 2 h: 5.10 ± 2.04, after 24 h: 4.97 ± 2.33 Control: Pre: 5.54 ± 2.07, after 2 h: 5.31 ± 2.00, after 24 h: 5.66 ± 2.16 (** p = 0.004) Plasma vitamin C increased after 2 h, marker for oxidative stress decreased in the verum group (** p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollbracht, C.; Kraft, K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients 2021, 13, 1154. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041154
Vollbracht C, Kraft K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients. 2021; 13(4):1154. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041154
Chicago/Turabian StyleVollbracht, Claudia, and Karin Kraft. 2021. "Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue" Nutrients 13, no. 4: 1154. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041154