Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor
Abstract
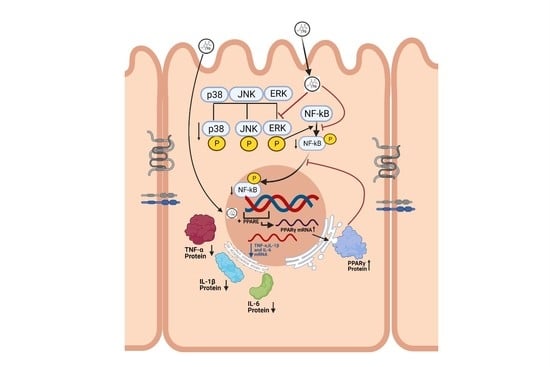
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Experimental Design
2.4. Evaluation of Disease Activity Index (DAI) Score
2.5. Proinflammatory Cytokines Estimation by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Myeloperoxidase (MPO) Assay
2.7. Histopathological Evaluation
2.8. RNA Extraction and Real-Time RT-PCR
2.9. Western Blot
2.10. HT-29 Cell Culture
2.11. Cell Viability Assay
2.12. PPAR-γ Promotor and Nanoluciferase Assay
2.13. Statistical Analysis
3. Results
3.1. Effect of TQ on Disease Activity Index (DAI), Colon Length, and Myeloperoxidase (MPO) Activity
3.2. Effect of TQ on Microscopic Architecture of the Inflamed Colon
3.3. Effect of TQ on Proinflammatory Cytokines and Mediators
3.4. Effect of TQ on MAPK Signaling Pathway and PPAR-γ Expression
3.5. Effect of TQ on TNF-α Treated HT-29 Cells and PPAR-γ Expression
3.6. Effect of TQ on PPAR-γ Promoter in HT-29 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Kalla, R.; Ho, G.T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Res 2020, 9, F1000 Faculty Rev-294. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Head, K.; Jurenka, J.S. Inflammatory bowel disease. Part II: Crohn’s disease--pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2004, 9, 360–401. [Google Scholar] [PubMed]
- Decara, J.; Rivera, P.; Lopez-Gambero, A.J.; Serrano, A.; Pavon, F.J.; Baixeras, E.; Rodriguez de Fonseca, F.; Suarez, J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front. Pharmacol. 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Caioni, G.; Viscido, A.; d’Angelo, M.; Panella, G.; Castelli, V.; Merola, C.; Frieri, G.; Latella, G.; Cimini, A.; Benedetti, E. Inflammatory Bowel Disease: New Insights into the Interplay between Environmental Factors and PPARgamma. Int. J. Mol. Sci. 2021, 22, 985. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, B.; Ojha, S.; Belur, P.D.; Bhongade, B.; Raj, V.; Collin, P.D.; Adrian, T.E.; Subramanya, S.B. Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor gamma in inflammatory bowel diseases. Phytother. Res. 2020, 34, 1530–1549. [Google Scholar] [CrossRef]
- Megantara, S.; Utami, D.; Puspitasari, L.; Mustarichie, R. Insilico Study of Thymoquinone as Peroxisome Proliferator Activated Receptor Gamma Agonist in the Treatment of Type 2 Diabetes Mellitus. J. Pharm. Sci. Res. 2017, 9, 1478. [Google Scholar]
- Woo, C.C.; Loo, S.Y.; Gee, V.; Yap, C.W.; Sethi, G.; Kumar, A.P.; Tan, K.H. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-gamma pathway. Biochem. Pharmacol. 2011, 82, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Elmaci, I.; Altinoz, M.A. Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomed. Pharm. 2016, 83, 635–640. [Google Scholar] [CrossRef]
- Noorbakhsh, M.F.; Hayati, F.; Samarghandian, S.; Shaterzadeh-Yazdi, H.; Farkhondeh, T. An Overview of Hepatoprotective Effects of Thymoquinone. Recent Pat. Food Nutr. Agric. 2018, 9, 14–22. [Google Scholar] [CrossRef] [PubMed]
- El-Dakhakhny, M.; Barakat, M.; El-Halim, M.A.; Aly, S.M. Effects of Nigella sativa oil on gastric secretion and ethanol induced ulcer in rats. J. Ethnopharmacol. 2000, 72, 299–304. [Google Scholar] [CrossRef]
- Magdy, M.A.; Hanan el, A.; Nabila el, M. Thymoquinone: Novel gastroprotective mechanisms. Eur. J. Pharmacol. 2012, 697, 126–131. [Google Scholar] [CrossRef]
- Broom, O.J.; Widjaya, B.; Troelsen, J.; Olsen, J.; Nielsen, O.H. Mitogen activated protein kinases: A role in inflammatory bowel disease? Clin. Exp. Immunol. 2009, 158, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Waetzig, G.H.; Seegert, D.; Rosenstiel, P.; Nikolaus, S.; Schreiber, S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J. Immunol. 2002, 168, 5342–5351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Afrose, S.S.; Junaid, M.; Akter, Y.; Tania, M.; Zheng, M.; Khan, M.A. Targeting kinases with thymoquinone: A molecular approach to cancer therapeutics. Drug Discov. Today 2020, 25, 2294–2306. [Google Scholar] [CrossRef]
- Lei, X.; Liu, M.; Yang, Z.; Ji, M.; Guo, X.; Dong, W. Thymoquinone prevents and ameliorates dextran sulfate sodium-induced colitis in mice. Dig. Dis. Sci. 2012, 57, 2296–2303. [Google Scholar] [CrossRef]
- Mahgoub, A.A. Thymoquinone protects against experimental colitis in rats. Toxicol. Lett. 2003, 143, 133–143. [Google Scholar] [CrossRef]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Subramanya, S.B.; Chandran, S.; Almarzooqi, S.; Raj, V.; Al Zahmi, A.S.; Al Katheeri, R.A.; Al Zadjali, S.A.; Collin, P.D.; Adrian, T.E. Frondanol, a Nutraceutical Extract from Cucumaria frondosa, Attenuates Colonic Inflammation in a DSS-Induced Colitis Model in Mice. Mar. Drugs 2018, 16, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, V.; Venkataraman, B.; Almarzooqi, S.; Chandran, S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; Subramanya, S.B. Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients 2020, 12, 2032. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Degrelle, S.A.; Shoaito, H.; Fournier, T. New Transcriptional Reporters to Quantify and Monitor PPARgamma Activity. PPAR Res 2017, 2017, 6139107. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Umar, S.; Hedaya, O.; Singh, A.K.; Ahmed, S. Thymoquinone inhibits TNF-alpha-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol. Appl. Pharmacol. 2015, 287, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef] [Green Version]
- Zobeiri, M.; Momtaz, S.; Parvizi, F.; Tewari, D.; Farzaei, M.H.; Nabavi, S.M. Targeting Mitogen-Activated Protein Kinases by Natural Products: A Novel Therapeutic Approach for Inflammatory Bowel Diseases. Curr. Pharm. Biotechnol. 2020, 21, 1342–1353. [Google Scholar] [CrossRef]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Kitanaka, N.; Nakano, R.; Sugiura, K.; Kitanaka, T.; Namba, S.; Konno, T.; Nakayama, T.; Sugiya, H. Interleukin-1beta promotes interleulin-6 expression via ERK1/2 signaling pathway in canine dermal fibroblasts. PLoS ONE 2019, 14, e0220262. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M.; et al. Deletion of IL-6 Exacerbates Colitis and Induces Systemic Inflammation in IL-10-Deficient Mice. J. Crohns. Colitis 2020, 14, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Assi, K.; Pillai, R.; Gomez-Munoz, A.; Owen, D.; Salh, B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology 2006, 118, 112–121. [Google Scholar] [CrossRef]
- Hollenbach, E.; Neumann, M.; Vieth, M.; Roessner, A.; Malfertheiner, P.; Naumann, M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004, 18, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Suzuki, A.; Tomiyasu, N.; Tsuruta, O.; Kitazaki, S.; Takeda, T.; Satoh, Y.; Bennett, B.L.; Toyonaga, A.; Sata, M. Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int. J. Mol. Med. 2006, 17, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qiao, J.; Zhao, X.; Chen, T.; Guan, D. Thymoquinone Inhibits IL-1beta-Induced Inflammation in Human Osteoarthritis Chondrocytes by Suppressing NF-kappaB and MAPKs Signaling Pathway. Inflammation 2015, 38, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Toyota, Y.; Nomura, S.; Makishima, M.; Hashimoto, Y.; Ishikawa, M. Structure-activity relationships of rosiglitazone for peroxisome proliferator-activated receptor gamma transrepression. Bioorg. Med. Chem. Lett. 2017, 27, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Zhao, H. Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-gamma and PI3K/Akt pathways. Exp. Ther. Med. 2018, 15, 4987–4994. [Google Scholar]
- Qadi, S.A.; Hassan, M.A.; Sheikh, R.A.; Baothman, O.A.; Zamzami, M.A.; Choudhry, H.; Al-Malki, A.L.; Albukhari, A.; Alhosin, M. Thymoquinone-Induced Reactivation of Tumor Suppressor Genes in Cancer Cells Involves Epigenetic Mechanisms. Epigenet. Insights 2019, 12, 2516865719839011. [Google Scholar] [CrossRef]
- Zweibaum, A.; Laburthe, M.; Grasset, E.; Louvard, D. Use of cultured cell lines in studies of intestinal cell differentiation and function. Compr. Physiol. 2010, 223–255. [Google Scholar]
- Bourgine, J.; Billaut-Laden, I.; Happillon, M.; Lo-Guidice, J.M.; Maunoury, V.; Imbenotte, M.; Broly, F. Gene expression profiling of systems involved in the metabolism and the disposition of xenobiotics: Comparison between human intestinal biopsy samples and colon cell lines. Drug Metab. Dispos. 2012, 40, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukahara, T.; Haniu, H. Peroxisome proliferator-activated receptor gamma overexpression suppresses proliferation of human colon cancer cells. Biochem. Biophys. Res. Commun. 2012, 424, 524–529. [Google Scholar] [CrossRef]
- Ponce de Leon-Rodriguez, M.D.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef]
- Attoub, S.; Sperandio, O.; Raza, H.; Arafat, K.; Al-Salam, S.; Al Sultan, M.A.; Al Safi, M.; Takahashi, T.; Adem, A. Thymoquinone as an anticancer agent: Evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Imada, A.; Ina, K.; Shimada, M.; Yokoyama, T.; Yokoyama, Y.; Nishio, Y.; Yamaguchi, T.; Ando, T.; Kusugami, K. Coordinate upregulation of interleukin-8 and growth-related gene product-alpha is present in the colonic mucosa of inflammatory bowel. Scand. J. Gastroenterol. 2001, 36, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Sekikawa, A.; Yamagishi, H.; Ichikawa, K.; Tomita, S.; Imura, J.; Ito, Y.; Fujita, M.; Tsubaki, M.; Kato, H.; et al. GROalpha promotes invasion of colorectal cancer cells. Oncol. Rep. 2010, 24, 1479–1486. [Google Scholar]
- Zhang, M.; Wang, G.; Tao, Y.; Zhang, H. The proinflammatory effect and molecular mechanism of IL- 17 in the intestinal epithelial cell line HT-29. J. BUON 2015, 20, 120–127. [Google Scholar] [PubMed]
- Ashour, A.E.; Abd-Allah, A.R.; Korashy, H.M.; Attia, S.M.; Alzahrani, A.Z.; Saquib, Q.; Bakheet, S.A.; Abdel-Hamied, H.E.; Jamal, S.; Rishi, A.K. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol. Cell Biochem. 2014, 389, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Weight Loss | Score | Stool Consistency | Score | Rectal Bleeding | Score |
|---|---|---|---|---|---|
| No loss | 0 | Normal | 0 | No Blood | 0 |
| 1–5% | 1 | Loose stool | 2 | Heme occult +ve and visual pellet bleeding | 2 |
| 5–10% | 2 | Diarrhea | 4 | Gross bleeding and blood around anus | 4 |
| 10–20% | 3 | ||||
| >20% | 4 |
| Inflammation Graded | Percentage of Inflammation Involvement of Mucosal Surface Area | Hyperplastic Epithelium Graded Based on Extent of Involvement | ||
|---|---|---|---|---|
| none | 0 | no inflammation | 0 | none |
| mild | 1 | 1–25% | 1 | 1–25% |
| moderate | 2 | 26–50% | 2 | 26–50% |
| severe | 3 | 51–75% | 3 | 51–75% |
| 4 | 76–100% | 4 | 76–100% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkataraman, B.; Almarzooqi, S.; Raj, V.; Alhassani, A.T.; Alhassani, A.S.; Ahmed, K.J.; Subramanian, V.S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; et al. Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor. Nutrients 2021, 13, 1343. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041343
Venkataraman B, Almarzooqi S, Raj V, Alhassani AT, Alhassani AS, Ahmed KJ, Subramanian VS, Ojha SK, Attoub S, Adrian TE, et al. Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor. Nutrients. 2021; 13(4):1343. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041343
Chicago/Turabian StyleVenkataraman, Balaji, Saeeda Almarzooqi, Vishnu Raj, Abdullah T. Alhassani, Ahmad S. Alhassani, Khadijah J. Ahmed, Veedamali S. Subramanian, Shreesh K. Ojha, Samir Attoub, Thomas E. Adrian, and et al. 2021. "Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor" Nutrients 13, no. 4: 1343. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041343