Cow’s Milk Protein Allergy as a Model of Food Allergies
Abstract
:1. Introduction
2. Cow’s Milk Composition
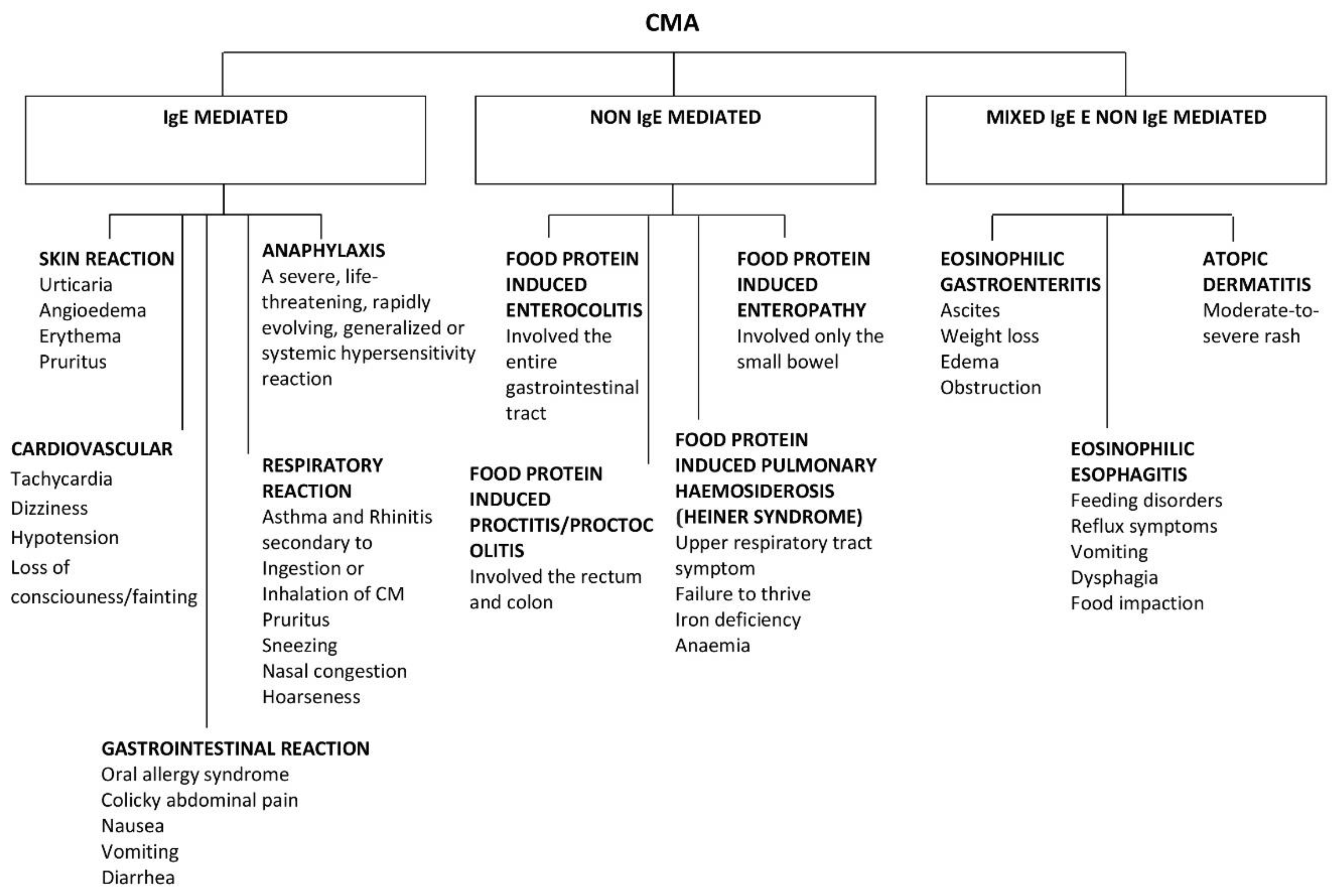
3. Subtypes of Immune-Mediated Reactions to CM
4. Prevalence of CMA
5. Diagnosis
6. Risk Factors for CMA
6.1. The Role of Vitamin D
6.2. The Role of Breastfeeding
7. Natural History of CMA
| Authors/Year of Publication | Number of Subjects | Population/Study Design | Tolerance Rate | Age of Tolerance(year) |
|---|---|---|---|---|
| Høst et al., 2002 [86] | 39 (24 IgE-mediated) | General prospective birth cohort | 56% | 1 |
| 77% | 2 | |||
| 87% | 3 | |||
| 92% | 5 | |||
| 97% | 15 | |||
| Vanto et al., 2004 [44] | 162 (95 IgE-mediated) | Referral retrospective | 44% | 2 |
| 69% | 3 | |||
| 77% | 4 | |||
| Garcia-Ara et al., 2004 [32] | 66 IgE-mediated | Referral retrospective | 68% | 4 |
| Saarinen et al., 2005 [85] | 118 (75 IgE-mediated) | General prospective birth cohort | 51% | 2 |
| 74% | 5 | |||
| 85% | 8.6 | |||
| Skripak et al., 2007 [84] | 807 IgE-mediated | Referral retrospective | 19% | 4 |
| 42% | 8 | |||
| 64% | 12 | |||
| 79% | 16 | |||
| Fiocchi et al., 2008 [87] | 112 IgE-mediated | Referral retrospective | 52.7% | 5 |
| Martorell et al., 2008 [34] | 170 IgE-mediated | Referral retrospective | 82% | 4 |
| Santos et al., 2010 [47] | 139 (66 IgE-mediated) | Referral retrospective | 41% | 2 |
| Ahrens et al., 2012 [88] | 52 IgE-mediated | Referral retrospective | 61.5% | 12 |
| Elizur et al., 2012 [83] | 54 IgE-mediated | General prospective birth cohort | 57.4% | 2 |
| 65% | 4 | |||
| Wood et al., 2013 [89] | 293 IgE-mediated | Prospective | 53% | 5.5 |
| Yavuz et al., 2013 [49] | 148 IgE-mediated | Referral retrospective | 20% | 2 |
| 34% | 4 | |||
| 39% | 6 | |||
| Schoemaker et al., 2015 [21] | 55 | EuroPrevall, European population-based | 57% | 2 |
| Prospective |
8. Factors Associated with the Natural History
The Role of Baked Milk
9. Treatment and Oral Immunotherapy
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousan, G.; Kamat, D. Cow’s milk protein allergy. Clin. Pediatr. 2016, 55, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Firer, M.A.; Shelton, M.J.; Hosking, C.S. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J. Pediatr. 1986, 109, 270–276. [Google Scholar] [CrossRef]
- Lomer, M.C.; Parkes, G.C.; Sanderson, J.D. Review article: Lactose intolerance in clinical practice—Myths and realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef]
- Burks, A.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 129906. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Savage, J.; Sicherer, S.; Wood, R. The natural history of food allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef]
- Wal, J.M. Bovine milk allergenicity. Ann. Allergy Asthma Immunol. 2004, 93, S2–S11. [Google Scholar] [CrossRef]
- Séverin, S.; Wenshui, X. Milk biologically active components as nutraceuticals: 546 review. Crit. Rev. food Sci. Nutr. 2005, 45, 645–656. [Google Scholar] [CrossRef]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Caffarelli, C.; Baldi, F.; Bendandi, B.; Calzone, L.; Marani, M.; Pasquinelli, P. Cow’s milk protein allergy in children: A practical guide. Ital. J. Pediatr. 2010, 36, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restani, P.; Ballabio, C.; Di Lorenzo, C.; Tripodi, S.; Fiocchi, A. Molecular aspects of milk allergens and their role in clinical events. Anal. Bioanal. Chem. 2009, 395, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and rationale for action against cow’s milk allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12. [Google Scholar] [CrossRef]
- Host, A. Cow’s milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr. Allergy Immunol. 1994, 5, 1–36. [Google Scholar] [CrossRef]
- Savage, J.; Johns, C.B. Food allergy epidemiology and natural history food allergy epidemiology natural history peanut milk egg. Immunol. Allergy Clin. NA 2015, 35, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Helyeh, S.; David, L.; Gary, S. Advances in the management of food allergy in children. Curr. Pediatr. Rev. 2018, 14, 150–155. [Google Scholar] [CrossRef]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 992–1007. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Kansu, A.; Yüce, A.; Dalgıç, B.; Şekerel, B.E.; Çullu-Çokuğraş, F.; Çokuğraş, H. Consensus statement on diagnosis, treatment and follow-up of cow’s milk protein allergy among infants and children in Turkey. Turk. J. Pediatr. 2016, 58, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, B.; Indirli, G.C.; Bianchi, A.; Arasi, S.; Caimmi, D.; Dondi, A.; La Grutta, S.; Panetta, V.; Verga, M.C.; Calvani, M. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow’s milk according to age: A systematic review. Ital. J. Pediatr. 2017, 43, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Komata, T.; Söderström, L.; Borres, M.P.; Tachimoto, H.; Ebisawa, M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J. Allergy Clin. Immunol. 2007, 119, 1272. [Google Scholar] [CrossRef]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, S.A.; Sicherer, S.H.; Teuber, S.S. Work group report: Oral food challenge testing. Adverse reactions to food committee of American academy of allergy, asthma & immunology. J. Allergy Clin. Immunol. 2009, 123 (Suppl. 6), S365–S383. [Google Scholar]
- Calatayud, C.M.; García, A.M.; Aragonés, A.M.; Caballer, B.D. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow’s milk allergy in children: Systematic review and meta-analysis. J. Investig. Allergol. Clin. Immunol. 2014, 24, 298–307. [Google Scholar]
- Sampson, H.A.; Ho, G. Clinical aspects of allergic disease Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J. Allergy Clin. Immunol. 1997, 100, 444–451. [Google Scholar] [CrossRef]
- García-Ara, C.; Boyano-Martínez, T.; Díaz-Pena, J.M.; Martín-Muñoz, F.; Reche-Frutos, M.; Martín-Esteban, M. Food and drug reactions and anaphylaxis Specific IgE levels in the diagnosis of immediate hypersensitivity to cow’s milk protein in the infant. J. Allergy Clin. Immunol. 2001, 107, 185–190. [Google Scholar] [CrossRef]
- Sampson, H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J. Allergy Clin. Immunol 2001, 107, 891–896. [Google Scholar] [CrossRef]
- García-Ara, M.C.; Boyano-Martínez, M.T.; Díaz-Pena, J.M.; Martín-Muñoz, M.F.; Martín-Esteban, M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef]
- Celik-Bilgili, S.; Mehl, A.; Verstege, A.; Staden, U.; Nocon, M.; Beyer, K.; Niggemann, B. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin. Exp. Allergy 2005, 35, 268–273. [Google Scholar] [CrossRef]
- Martorell, A.; Plaza, A.M.; Nevot, S.; Echeverria, L.; Alonso, E.; Garde, J. The predictive value of specific immunoglobulin E levels in serum for the outcome of the development of tolerance in cow’s milk allergy. Allergol. Immunopathol. 2008, 36, 325–330. [Google Scholar] [CrossRef]
- Van der Gugten, A.C.; den Otter, M.; Meijer, Y.; Pasmans, S.G.A.M.; Knulst, A.C.; Hoekstra, M.O. Usefulness of specific IgE levels in predicting cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 121, 531–533. [Google Scholar] [CrossRef]
- Ott, H.; Baron, J.M.; Heise, R.; Ocklenburg, C.; Stanzel, S.; Merk, H.F.; Niggemann, B.; Beyer, K. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy 2008, 63, 1521–1528. [Google Scholar] [CrossRef]
- Mehl, A.; Verstege, A.; Staden, U.; Kulig, M.; Nocon, M.; Beyer, K.; Niggemann, B. Utility of the ratio of food-specific IgE/total IgE in predicting symptomatic food allergy in children. Allergy 2005, 60, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Onesimo, R.; Monaco, S.; Greco, M.; Caffarelli, C.; Calvani, M.; Tripodi, S.; Sopo, S.M. Predictive value ofMP4 (Milk prick four), a panel of skin prick test for the diagnosis of pediatric immediate cow’s milk allergy. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 201–208. [Google Scholar] [PubMed]
- Eigenmann, P.A.; Sampson, H.A. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol. 1998, 9, 186–191. [Google Scholar] [CrossRef]
- Sporik, R.; Hill, D.J.; Hosking, C.S. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin. Exp. Allergy 2000, 30, 1540–1546. [Google Scholar] [CrossRef]
- Calvani, M.; Alessandri, C.; Frediani, T.; Lucarelli, S.; Miceli Sopo, S.; Panetta, V.; Zappalã, D.; Zicari, A.M. Correlation between skin prick test using commercial extract of cow’s milk protein and fresh milk and food challenges. Pediatr. Allergy Immunol. 2007, 18, 583–588. [Google Scholar]
- Calvani, M.; Berti, I.; Fiocchi, A.; Galli, E.; Giorgio, V.; Martelli, A.; Miceli Sopo, S.; Panetta, V. Oral food challenge: Safety, adherence to guidelines and predictive value of skin prick testing. Pediatr Allergy Immunol. 2012, 23, 755–761. [Google Scholar] [CrossRef]
- Kido, J.; Hirata, M.; Ueno, H.; Nishi, N.; Mochinaga, M.; Ueno, Y.; Yanai, M.; Johno, M.M. Evaluation of the skin-prick test for predicting the outgrowth of cow’s milk allergy. Allergy Rhinol. 2016, 7, 39–143. [Google Scholar] [CrossRef]
- Vanto, T.; Helppilä, S.; Juntunen-Backman, K.; Kalimo, K.; Klemola, T.; Korpela, R.; Koskinen, P. Prediction of the development of tolerance to milk in children with cow’s milk hypersensitivity. J. Pediatr. 2004, 144, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Cow’s milk protein-specific IgE concentrations in two age groups of milk-allergic children and in children achieving clinical tolerance. Clin. Exp. Allergy 1999, 29, 507–512. [Google Scholar] [CrossRef]
- Shek, L.P.C.; Soderstrom, L.; Ahlstedt, S.; Beyer, K.; Sampson, H.A. Determination of food specific IgE levels over time can predict the development of tolerance in cow’s milk and hen’s egg allergy. J. Allergy Clin. Immunol. 2004, 114, 387–391. [Google Scholar] [CrossRef]
- Santos, A.; Dias, A.; Pinheiro, J.A. Predictive factors for the persistence of cow’s milk allergy. Pediatr. Allergy Immunol. 2010, 21, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C. How to reintroduce cow’s milk? Pediatr. Allergy Immunol. 2013, 24, 627–632. [Google Scholar] [CrossRef]
- Yavuz, S.T.; Buyuktiryaki, B.; Sahiner, U.M.; Birben, E.; Tuncer, A.; Yakarisik, S.; Karabulut, E.; Kalayci, O.; Sackesen, C. Factors that predict the clinical reactivity and tolerance in children with cow’s milk allergy. Ann. Allergy Asthma Immunol. 2013, 110, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Allen, K.J.; Gurrin, L.C.; Peters, R.L.; Healthnuts, T.; Team, S.; Ponsonby, A.; Hill, D.; Matheson, M.; Wake, M.; et al. The impact of family history of allergy on risk of food allergy: A population-based study of infants. Int. J. Environ. Res. Public Health 2013, 10, 5364–5377. [Google Scholar] [CrossRef]
- Goldberg, M.; Eisenberg, E.; Elizur, A.; Rajuan, N.; Rachmiel, M.; Cohen, A.; Zadik-Mnuhin, G.; Katz, Y. Role of parental atopy in cow’s milk allergy: A population-based study. Ann. Allergy Asthma Immunol. 2013, 110, 279–283. [Google Scholar] [CrossRef]
- Sardecka, I.; Los-Rycharska, E.; Ludwig, H.; Gawryjołek, J.; Krogulska, A. Early risk factors for cow’s milk allergy in children in the first year of life. Allergy Asthma Proc. 2018, 39, e44–e54. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Hosking, C.S. Food allergy and atopic dermatitis in infancy: An epidemiologic study. Pediatr. Allergy Immunol. 2004, 15, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Boyano-Martinez, T.; Garcia-Ara, C.; Pedrosa, M.; Diaz-Pena, J.M.; Quirce, S. Accidental allergic reactions in children allergic to cow’s milk proteins. J. Allergy Clin. Immunol. 2009, 123, 883–888. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Wood, R.A.; Matsui, E.C. Personal and parental nativity as risk factors for food sensitization. J. Allergy Clin. Immunol. 2012, 129, 169–175.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering and Medicine. Finding a Path to Safety in Food Allergy: Assessment of Global Burden, Causes, Prevention, Management, and Public Policy; National Academies of Sciences: Washington, DC, USA, 2016. [Google Scholar]
- Miyazawa, T.; Itabashi, K.; Imai, T. Retrospective multicenter survey on food-related symptoms suggestive of cow’s milk allergy in NICU neonates. Allergol Int. 2013, 62, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Iwakura, H.; Ohtsuka, H.; Kohno, Y.; Shimojo, N. Milk allergy in the neonatal intensive care unit: Comparison between premature and full-term neonates. Asia Pac. Allergy. 2013, 3, 335–341. [Google Scholar] [CrossRef]
- Edwards, M.O.; Kotecha, S.J.; Lowe, J.; Richards, L.; Watkins, W.J.; Kotecha, S. Early-term birth is a risk factor for wheezing in childhood: A cross- sectional population study. J. Allergy Clin. Immunol. 2015, 136, 581–587.e2. [Google Scholar] [CrossRef] [PubMed]
- Kvenshagen, B.; Halvorsen, R.; Jacobsen, M. Is there an increased frequency of food allergy in children delivered by caesarean section compared to those delivered vaginally? Acta Paediatr. 2009, 98, 324–327. [Google Scholar] [CrossRef] [PubMed]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Rochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the immune system: Genomic and non-genomic actions. Mini Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G. (Brad) Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharief, S.; Jariwala, S.; Kumar, J.; Muntner, P.; Melamed, M.L. Vitamin D levels and food and environmental allergies in the United States: Results from NHANES 2005–2006. J. Allergy Clin. Immunol. 2011, 127, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, C.; Passalacqua, G. Vitamin D levels and allergic diseases. An Italian cross-sectional multicenter survey. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 75–79. [Google Scholar]
- Giannetti, A.; Bernardini, L.; Cangemi, J.; Gallucci, M.; Masetti, R.; Ricci, G. Role of Vitamin D in prevention of food allergy in infants. Front. Pediatr. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Fiocchi, A.; Dahda, L.; Dupont, C.; Campoy, C.; Fierro, V.; Nieto, A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ. J. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, F.; Comberiati, P.; Munblit, D.; Piacentini, G.L.; Antoniazzi, E.; Zanoni, L.; Boner, A.L.; Peroni, D.G. Breast-milk characteristics protecting against allergy. Endocr Metab Immune Disord Drug Targets 2014, 14, 9–15. [Google Scholar] [CrossRef]
- Verduci, E.; D’elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s milk substitutes for children: Nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [Green Version]
- Department of Child Adolescent Health Development. The optimal duration of exclusive breastfeeding. In Report of an Expert Consultation. Department of Nutrition for Health and Development; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Matangkasombut, P.; Padungpak, S.; Thaloengsok, S.; Kamchaisatian, W.; Sasisakulporn, C.; Jotikasthira, W.; Manuyakorn, W. Paediatrics and International child health detection of β-lactoglobulin in human breast-milk 7 days after cow milk ingestion. Paediatr. Int. Child. Health 2017, 37, 199–203. [Google Scholar] [CrossRef]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Grimshaw, K.E.; Maskell, J.; Oliver, E.M.; Morris, R.C.; Foote, K.D.; Mills, E.N.; Roberts, G.M. Introduction of complementary foods and the relationship to food allergy. Pediatrics 2013, 132, e1529–e1538. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US); Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. Comparing Infant Formulas with Human Milk; National Academies Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition, & Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: The Role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [Green Version]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; American Academy of Pediatrics Committee on Nutrition, & American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarinen, K.M.; Juntunen-Backman, K.; Järvenpää, A.L.; Kuitunen, P.; Lope, L.; Renlund, M.; Siivola, M.; Savilahti, E. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J. Allergy Clin. Immunol. 1999, 104, 457–461. [Google Scholar] [CrossRef]
- Høst, A.; Husby, S.; Osterballe, O. A prospective study of cow s milk allergy in exclusively breast-fed infants. Acta Paediatr. Scand. 1988, 77, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Rajuan, N.; Goldberg, M.R.; Eisenberg, E.; Heyman, E.; Cohen, A.; Leshno, M. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J. Allergy Clin. Immunol. 2010, 126, 77–82.e1. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, Y.; Noguchi, E.; Okada, M.; Sumazaki, R. The Association of the delayed introduction of cow’s milk with IgE-mediated cow’s milk allergies. J. Allergy Clin. Immunol. Pract. 2021, 4, 481–488.e2. [Google Scholar] [CrossRef] [PubMed]
- Elizur, A.; Rajuan, N.; Goldberg, M.R.; Leshno, M.; Cohen, A.; Katz, Y. Natural course and risk factors for persistence of IgE-mediated cow’s milk allergy. YMPD 2012, 161, 482–487.e1. [Google Scholar] [CrossRef]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef]
- Saarinen, K.M.; Pelkonen, A.S.; Mäkelä, M.J.; Savilahti, E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J. Allergy Clin. Immunol. 2005, 116, 869–875. [Google Scholar] [CrossRef]
- Høst, A.; Halken, S.; Jacobsen, H.P.; Christensen, A.E.; Herskind, A.M.; Plesner, K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. 2002, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Terracciano, L.; Bouygue, G.R.; Veglia, F.; Sarratud, T.; Martelli, A.; Restani, P. Incremental prognostic factors associated with cow’s milk allergy outcomes in infant and child referrals: the Milan Cow’s Milk Allergy Cohort study. Annals of allergy, asthma & immunology: Official publication of the American College of Allergy. Asthma Immunol. 2008, 101, 166–173. [Google Scholar]
- Ahrens, B.; Lopes de Oliveira, L.C.; Grabenhenrich, L.; Schulz, G.; Niggemann, B.; Wahn, U.; Beyer, K. Individual cow’s milk allergens as prognostic markers for tolerance development? Clin. Exp. Allergy 2012, 42, 1630–1637. [Google Scholar] [CrossRef]
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Nowak-Wegrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J. Allergy Clin. Immunol. 2011, 128, 125–131.e2. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Wood, R.A.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Dawson, P.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of egg allergy in an observational cohort. J. Allergy Clin. Immunol. 2015, 133, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Lee, Y.; Yang, H.; Won, J. The natural course of immediate-type cow’s milk and egg allergies in children. Int. Arch. Allergy Immunol. 2020, 181, 103–110. [Google Scholar] [CrossRef]
- Perry, T.T.; Matsui, E.C.; Conover-Walker, M.K.; Wood, R.A. The relationship of allergen-specific IgE levels and oral food challenge outcome. J. Allergy Clin. Immunol. 2004, 114, 144–149. [Google Scholar] [CrossRef]
- Leonard, S.A.; Caubet, J.C.; Kim, J.S.; Groetch, M.; Nowak-Wegrzyn, A. Baked milk-and egg-containing diet in the management of milk and egg allergy. J. Allergy Clin. Immunol. Pract. 2015, 3, 13–23. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s milk allergy: Immunomodulation by dietary intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uncuoglu, A.; Yologlu, N.; Simsek, I.E.; Uyan, Z.S.; Aydogan, M. Tolerance to baked and fermented cow’s milk in children with IgE-mediated and non-IgE-mediated cow’s milk allergy in patients under two years of age. Allergol. Immunopathol. 2017, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347.e2. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr. Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Host, A.; Halken, S. Cow’s milk allergy: Where have we come from and where are we going? Endocrine Metab. Immune Disord. Targets 2014, 14, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Brotons-Canto, A.; Martín-Arbella, N.; Gamazo, C.; Irache, J.M. New pharmaceutical approaches for the treatment of food allergies. Expert Opin. Drug Deliv. 2018, 15, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Alonso Lebrero, E.; Fernández Moya, L.; Somoza Álvarez, M.L. Sesión de actualización alergia a alimentos en niños. Alergol. Inmunol. Clin. 2001, 16, 96–115. [Google Scholar]
- Alonso Lebrero, E.; Fernández, L.S. Alergia a leche y huevo en niños. Alergol. Inmunol. Clin. 2001, 6, 96–110. [Google Scholar]
- Zapatero, L.; Alonso, E.; Fuentes, V.; Martínez, M.I. Oral desensitization in children with cow’s milk allergy. J. Investig. Allergol. Clin. Immunol. 2008, 18, 389–396. [Google Scholar]
- Mestecky, J.; McGhee, J.R.; Bienenstock, J.; Lamm, M.E.; Strober, W.; Cebra, J.J.; Mayer, L.; Ogra, P.L.; Russell, M.W. Historical aspects of mucosal immunology. In Mucosal Immunology, 4th ed.; Nature: Oberhaching, Germany, 1983; pp. 37–51. [Google Scholar]
- Noimark, L.; Cox, H.E. Nutritional problems related to food allergy in childhood. Pediatr. Allergy Immunol. 2008, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.K.; Martino, D.J. Oral immunotherapy and tolerance induction in childhood. Pediatr. Allergy Immunol. 2013, 24, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Barni, S.; Liccioli, G.; Novembre, E. Oral immunotherapy (OIT): A personalized medicine. Medicina 2019, 55, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Garcıa, S.; Rodríguez del Río, P.; Escudero, C.; Garcıa-Fernandez, C.; Ramirez, A.; Ibáñez, M.D. Efficacy of oral immunotherapy protocol for specific oral tolerance induction in children with cow’s milk allergy. ISR Med. Assoc. J. 2012, 14, 43–47. [Google Scholar] [PubMed]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [Green Version]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 799–815. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-García, S.; Cipriani, F.; Ricci, G. Food allergy in childhood: Phenotypes, prevention and treatment. Pediatr. Allergy Immunol. 2015, 26, 711–720. [Google Scholar] [CrossRef]
- Staden, U.; Rolinck-Werninghaus, C.; Brewe, F.; Wahn, U.; Niggemann, B.; Beyer, K. Specific oral tolerance induction in food allergy in children: Efficacy and clinical patterns of reaction. Allergy 2007, 62, 1261–1269. [Google Scholar] [CrossRef]
- Barni, S.; Mori, F.; Piccorossi, A.; Sarti, L.; Pucci, N.; Maresca, M.; Giovannini, M.; Liccioli, G.; Novembre, E. Low-Dose oral food challenge with hazelnut: E_cacy and tolerability in children. Int. Arch. Allergy Immunol. 2019, 178, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Caminiti, L.; Salzano, G.; Crisafulli, G.; Aversa, T.; Messina, M.F.; Wasniewska, M.; Passalacqua, G. Comparison between two maintenance feeding reimens after successful cow’s milk oral desensitization. Pediatr. Allergy Immunol. 2013, 24, 376–381. [Google Scholar] [CrossRef]
- Caminiti, L.; Passalacqua, G.; Barberi, S.; Vita, D.; Barberio, G.; De Luca, R.; Pajno, G.B. A new protocol for specific oral tolerance induction in children with IgE-mediated cow’s milk allergy. Allergy Asthma Proc. 2009, 30, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Sato, S.; Fiocchi, A.; Ebisawa, M. Oral and sublingual immunotherapy for food allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Meglio, P.; Bartone, E.; Plantamura, M.; Arabito, E.; Giampietro, P.G. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004, 59, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Narisety, S.D.; Skripak, J.M.; Steele, P.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2009, 124, 610–612. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.R.; Nachshon, L.; Appel, M.Y.; Elizur, A.; Levy, M.B.; Eisenberg, E.; Sampson, H.A.; Katz, Y. Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J. Allergy Clin. Immunol. 2015, 136, 1601–1606. [Google Scholar] [CrossRef]
- Takahashi, M.; Taniuchi, S.; Soejima, K.; Hatano, Y.; Yamanouchi, S.; Kaneko, K. Two-weeks-sustained unresponsiveness by oral immunotherapy using microwave heated cow’s milk for children with cow’s milk allergy. Allergy Asthma Clin. Immunol. 2016, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Gharagozlou, M.; Mohebbi, A.; Hafezi, N.; Azizi, G.; Brahimi, M.; Gharagozlou, M.; Mohebbi, A.; Hafezi, N.; Azizi, G.; et al. The efficacy of oral immunotherapy in patients with cow’s milk allergy. Iran. J. Allergy Asthma Immunol. 2017, 16, 183–192. [Google Scholar]
- Skripak, J.M.; Nash, S.D.; Rowley, H.; Brereton, N.H.; Oh, S.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Longo, G.; Barbi, E.; Berti, I.; Meneghetti, R.; Pittalis, A.; Ronfani, L.; Ventura, A. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J. Allergy Clin. Immunol. 2008, 121, 343–347. [Google Scholar] [CrossRef]
- Pajno, G.B.; Caminiti, L.; Ruggeri, P.; De Luca, R.; Vita, D.; La Rosa, M.; Passalacqua, G. Oral immunotherapy for cow’s milk allergy with a weekly up-dosing regimen: A randomized single-blind controlled study. Ann. Allergy Asthma Immunol. 2010, 105, 376–381. [Google Scholar] [CrossRef]
- Martorell, A.; De la Hoz, B.; Ibáñez, M.D.; Bone, J.; Terrados, M.S.; Michavila, A.; Plaza, A.M.; Alonso, E.; Garde, J.; Nevot, S.; et al. Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin. Exp. Allergy. 2011, 41, 1297–1304. [Google Scholar] [CrossRef]
- Amat, F.; Kouche, C.; Gaspard, W.; Lemoine, A.; Guiddir, T.; Lambert, N.; Zakariya, M.; Ridray, C.; Nemni, A.; Saint-Pierre, P.; et al. Is a slow-progression baked milk protocol of oral immunotherapy always a safe option for children with cow’s milk allergy? A randomized controlled trial. Clin. Exp. Allergy. 2017, 47, 1491–1496. [Google Scholar] [CrossRef]
- Maeda, M.; Imai, T.; Ishikawa, R.; Nakamura, T.; Kamiya, T.; Kimura, A.; Fujita, S.; Akashi, K.; Tada, H.; Morita, H.; et al. Effect of oral immunotherapy in children with milk allergy: The ORIMA study. Allergol Int. 2021, 70, 223–228. [Google Scholar] [CrossRef]
- Mota, I.; Piedade, S.; Gaspar, Â.; Benito-Garcia, F.; Sampaio, G.; Borrego, L.M.; Morais-Almeida, M. Cow’s milk oral immunotherapy in real life: 8-year long-term follow-up study. Asia Pac. Allergy 2018, 8, e28. [Google Scholar] [CrossRef]
- Berti, I.; Badina, L.; Cozzi, G.; Giangreco, M.; Bibalo, C.; Ronfani, L.; Barbi, E.; Ventura, A.; Longo, G. Early oral immunotherapy in infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2019, 30, 572–574. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, S.; Mazer, B.; Clarke, A.E.; Pierre, Y.S.; Lejtenyi, D. Adverse events in oral immunotherapy for the desensitization of cow’s milk allergy in children: A randomized controlled trial. J. Allergy Clin. Immunol. Pract. 2019, 7, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Efron, A.; Zeldin, Y.; Gotesdyner, L.; Stauber, T.; Maoz Segal, R.; Binson, I.; Dinkin, M.; Dinkowitz, L.; Shahar, D.; Deutch, M.; et al. A structured gradual exposure protocol to baked and heated milk in the treatment of milk allergy. J. Pediatr. 2018, 203, 204–209. [Google Scholar] [CrossRef]
- Kauppila, T.K.; Paassilta, M.; Kukkonen, A.K.; Kuitunen, M.; Pelkonen, A.S.; Makela, M.J. Outcome of oral immunotherapy for persistent cow’s milk allergy from 11 years of experience in Finland. Pediatr. Allergy Immunol. 2019, 30, 356–362. [Google Scholar] [CrossRef]
- Demir, E.; Ciğerci Günaydın, N.; Gülen, F.; Tanaç, R. Oral Immunotherapy for cow’s milk allergy: five years’ experience from a single center in Turkey. Balk. Med. J. 2020, 37, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Gruzelle, V.; Juchet, A.; Martin-Blondel, A.; Michelet, M.; Chabbert-Broue, A.; Didier, A. Benefits of baked milk oral immunotherapy in French children with cow’s milk allergy. Pediatr. Allergy Immunol. 2020, 31, 364–370. [Google Scholar] [CrossRef]
- Morisset, M.; Moneret-Vautrin, D.A.; Guenard, L.; Cuny, J.M.; Frentz, P.; Hatahet, R.; Hanss, C.H.; Beaudouin, E.; Petit, N.; Kanny, G. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 12–19. [Google Scholar] [PubMed]
- Patriarca, G.; Nucera, E.; Roncallo, C.; Pollastrini, E.; Bartolozzi, F.; De Pasquale, T.; Buonomo, A.; Gasbarrini, G.; Di Campli, C.; Schiavino, D. Oral desensitizing treatment in food allergy: Clinical and immunological results. Aliment. Pharmacol. Ther. 2003, 17, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A. Oral immunotherapy for food allergy. J. Investig. Allergol. Clin. Immunol. 2017, 27, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy 2017, 72, 1133–1147. [Google Scholar] [CrossRef]
- Epstein-Rigbi, N.; Goldberg, M.R.; Levy, M.B.; Nachshon, L.; Elizur, A. Quality of life of children aged 8-12 years undergoing food allergy oral immunotherapy: Child and parent perspective. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2623–2632. [Google Scholar] [CrossRef]
- Carraro, S.; Frigo, A.C.; Perin, M.; Stefani, S.; Cardarelli, C.; Bozzetto, S.; Baraldi, E.; Zanconato, S. Impact of oral immunotherapy on quality of life in children with cow milk allergy: A pilot study. Int. J. Immunopathol. Pharmacol. 2012, 25, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Kauppila, T.K.; Pelkonen, A.S.; Roine, R.P.; Paassilta, M.; Kukkonen, K.; Sintonen, H.; Mäkelä, M. Health-related quality of life in patients who had partaken in milk oral immunotherapy and comparison to the general population. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 387–390. [Google Scholar] [CrossRef]
| Allergen Name | Protein | Concentration (g/L) | Size (kDa) | Prevalence (% of Patients) | Allergenic Activity (% of Patients) | |
|---|---|---|---|---|---|---|
| Whey (20%) (5 g/L) | Bos d 4 | α–Lactalbumin | 1–1.5 | 14.2 | 0–67 | 12 |
| Bos d 5 | β–Lactoglobulin | 3–4 | 18.3 | 13–62 | 19 | |
| Bos d 6 | Bovine serum albumin | 0.1–0.4 | 66.3 | 0–76 | 1 | |
| Bos d 7 | Immunoglobulins | 0.6–1 | 160 | 12–36 | ||
| Lactoferrin | 0.09 | 80 | 0–35 | 3 | ||
| Whole casein (80%) (30 g/L) | Bos d 9 | αS1–casein | 12–15 | 23.6 | 65–100 | 26 |
| Bos d 10 | αS2–casein | 3–4 | 25.2 | |||
| Bos d 11 | β–casein | 9–11 | 24 | 35–44 | 35 | |
| Bos d 12 | k–casein | 3–4 | 19 | 35–41 | 26 |
| Author, Year | Age | 90% | 95% | Method |
|---|---|---|---|---|
| Sampson and Ho, 1997 [29] | 5.2 years | 23 kU/L | 32 kU/L | CAP system FEIA |
| Garcia–Ara et al., 2001 [30] | 4.8 months | 2.5 kU/L | 5 kU/L | CAP system FEIA |
| Sampson, 2001 [31] | 3.8 years | 15 kU/L | 32 kU/L | CAP system FEIA |
| Garcia–Ara et al., 2004 [32] | 13–18 months | 1.5 kU/L | 2.7 kU/L | CAP system FEIA |
| 19–24 months | 6 kU/L | 9 kU/L | ||
| 25–36 months | 14 kU/L | 24 kU/L | ||
| Celik–Bilgili et al., 2005 [33] | <1 year | 25.8 kU/L | CAP system FEIA | |
| Komata et al., 2007 [25] | <1 year | 5.8 kU/L | CAP system FEIA | |
| 1 year | 38.6 kU/L | |||
| 2 years | 57.3 kU/L | |||
| Martorell et al., 2008 [34] | 12 months | 5.8 kU/L | CAP system FEIA | |
| 18 months | 9.8 kU/L | |||
| 24 months | 27.5 kU/L | |||
| 36 months | 7.4 kU/L | |||
| 48 months | 5 kU/L | |||
| Van der Gutgen et al., 2008 [35] | <2.5 years | 5 kU/L | 7.5 kU/L | CAP system FEIA |
| Ott. et al., 2008 [36] | 52.7 kU/L | 66.9 kU/L | CAP system FEIA |
| Author, Year | Age | Ø SPT 90% | Ø SPT 95% | Type of Allergen |
|---|---|---|---|---|
| Eigenmann and Sampson, 1998 [39] | 4.6 years | >5 mm | Glycerinate extract | |
| Sporik et al., 2000 [40] | 3 years | >8 mm | Glycerinate extract | |
| <2 years | >6 mm | |||
| Calvani et al., 2007 [41] | 3.6 years | 15 mm | Fresh milk | |
| 12 mm | α-Lactalbumin | |||
| 8 mm | Casein | |||
| 10 mm | β-lactoglobulin | |||
| Calvani et al., 2012 [42] | 3.7 years | 20 mm | Fresh milk | |
| 10 mm | α-Lactalbumin | |||
| 7 mm | Casein | |||
| 8 mm | β-Lactoglobulin | |||
| Onesimo et al., 2013 [38] | 2.7 years | 4.9 mm | α-Lactalbumin | |
| 4.3 mm | Casein | |||
| 5.6 mm | β-Lactaglobulin | |||
| Kido et al., 2016 [43] | 1.4 years | 15 mm | Glycerinate extract |
| Author, Year | Type of Study | Type of Milk | Population (n) | Age (years) | Partial Tolerance | Complete Tolerance |
|---|---|---|---|---|---|---|
| Meglio P. et al., 2004 [117] | Open-label | Fresh CM | 21 | 6–10 | 14.3% (40–80 mL of CM) | 71.4% (200 mL of CM) |
| Narisety SD. et al., 2009 [118] | Open | Fresh CM | 15 | 6–16 | 33% (16 g of CM proteins) | |
| Goldberg M. et al., 2015 [119] | Open | Baked CM | 14 | 6.5–12.7 | 21% (1.3 g of CM proteins) | |
| Takahashi M. et al. 2016 [120] | Open | Microwave heated CM | 31 | 5–17 | 45.2% (200 mL of CM) | |
| Ebrahimi M. et al.2017 [121] | Open | Fresh CM | 14 | 3.5–7 | 92.9% (200 to 250 mL of CM) | |
| Skripak et al., 2008 [122] | Randomized, double-blind, placebo-controlled | Fresh CM | 13 | 6–17 | 30.8% (500 mg of CM proteins) | |
| Longo G. et al., 2008 [123] | Randomized open-label | Fresh CM | 30 | 5–17 | 54% (5–150 mL of CM) | 36% (> 150 mL of CM) |
| Pajno GB. et al., 2010 [124] | Randomized, placebo controlled | Fresh CM | 15 | 4–10 | 67% (200 mL ofCM) | |
| Martorell A. et al., 2011 [125] | Randomized, placebo controlled | Fresh CM | 30 | 2–3 | 90% (200 mL of CM) | |
| Amat F. et al.2017 [126] | Randomized | Baked CMFresh CM | 43 | 3–10 | 26.8% (0.27–2.5 g of CM proteins) | 36.6% (2.72 g of CM proteins) |
| Maeda M, et al., 2020 [127] | Randomized controlled | Fresh CM | 28 | 3–12 | 50% (100 mL of CM) | |
| Mota I. et al., 2018 [128] | Prospective | Fresh CM | 42 | 2–18 | 92% (200 mL of CM) | |
| Berti I. et al., 2019 [129] | Prospective | Fresh CM | 73 | 3–11 | 97% (150 mL of CM) | |
| De Schryver S. et al., 2019 [130] | Prospective, randomized-controlled | Fresh CM | 41 | 6–18 | 73.2% (200 mL of CM) | |
| Efron A. et al.,2018 [131] | Retrospective, case-control | Fresh CM | 43 | 1–4 | 86% (250 mL of CM) | |
| Kauppila T.K. et al., 2019 [132] | Retrospective | Fresh CM | 296 | 5–17 | 56% (200 mL of CM) | |
| Demir E. et al., 2020 [133] | Retrospective, cohort study | Fresh CM | 47 | 3–13 | 89.3% (200 mL of CM) | |
| Gruzelle V. et al., 2020 [134] | Retrospective | Baked CM | 64 | 2–16 | 42.2% (254 mL of CM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13, 1525. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051525
Giannetti A, Toschi Vespasiani G, Ricci G, Miniaci A, di Palmo E, Pession A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients. 2021; 13(5):1525. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051525
Chicago/Turabian StyleGiannetti, Arianna, Gaia Toschi Vespasiani, Giampaolo Ricci, Angela Miniaci, Emanuela di Palmo, and Andrea Pession. 2021. "Cow’s Milk Protein Allergy as a Model of Food Allergies" Nutrients 13, no. 5: 1525. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051525






