Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery
Abstract
:1. Introduction
2. The Surgical Cascade
3. Surgical Role of Carbohydrate and Protein
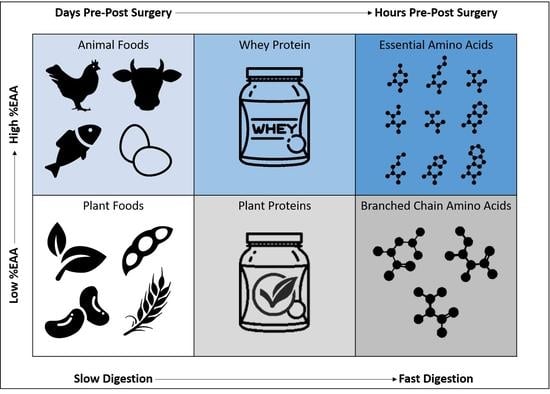
4. Nutrient Timing
5. Pre-Operative Nutrition
6. Post-Operative Nutrition
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wischmeyer, P.E.; Carli, F.; Evans, D.C.; Guilbert, S.; Kozar, R.; Pryor, A.; Thiele, R.H.; Everett, S.; Grocott, M.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy Within a Surgical Enhanced Recovery Pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Thomas, M.N.; Kufeldt, J.; Kisser, U.; Hornung, H.-M.; Hoffmann, J.; Andraschko, M.; Werner, J.; Rittler, P. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G-DRG-system. Nutrition 2016, 32, 249–254. [Google Scholar] [CrossRef]
- Geurden, B.; Franck, E.; Weyler, J.; Ysebaert, D. The risk of malnutrition in community-living elderly on admission to hospital for major surgery. Acta Chir. Belg. 2015, 115, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Corish, C.A.; Kennedy, N.P. Protein–energy undernutrition in hospital in-patients. Br. J. Nutr. 2000, 83, 575–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, S.E.; Hilkewich, L.; Gillis, C.; Heine, J.A.; Fenton, T.R. Protein intakes are associated with reduced length of stay: A comparison between Enhanced Recovery After Surgery (ERAS) and conventional care after elective colorectal surgery. Am. J. Clin. Nutr. 2017, 106, 44–51. [Google Scholar] [CrossRef]
- Aucoin, S.; McIsaac, D.I. Emergency General Surgery in Older Adults: A Review. Anesthesiol. Clin. 2019, 37, 493–505. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis, C.; Wischmeyer, P.E. Pre-operative nutrition and the elective surgical patient: Why, how and what? Anaesthesia 2019, 74 (Suppl. 1), 27–35. [Google Scholar] [CrossRef] [Green Version]
- Demling, R.H. Nutrition, anabolism, and the wound healing process: An overview. Eplasty 2009, 9, e9. [Google Scholar] [PubMed]
- Cartwright, M.M. The metabolic response to stress: A case of complex nutrition support management. Crit. Care Nurs. Clin. N. Am. 2004, 16, 467–487. [Google Scholar] [CrossRef]
- Gillis, C.; Carli, F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [Green Version]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Finney, S.J.; Zekveld, C.; Elia, A.; Evans, T.W. Glucose control and mortality in critically ill patients. JAMA 2003, 290, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Kilroe, S.P.; Fulford, J.; Jackman, S.R.; LJC, V.A.N.L.; Wall, B.T. Temporal Muscle-specific Disuse Atrophy during One Week of Leg Immobilization. Med. Sci. Sports Exerc. 2020, 52, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Strycker, L.A.; Senesac, H.A.; Hocker, A.D.; Smolkowski, K.; Shah, S.N.; Jewett, B.A. Essential amino acid supplementation in patients following total knee arthroplasty. J. Clin. Invest. 2013, 123, 4654–4666. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, H.C.; Owen, E.C.; Strycker, L.A.; Smolkowski, K.; Muyskens, J.B.; Kirkpatrick, T.K.; Christie, A.D.; Kuehl, K.S.; Lantz, B.A.; Shah, S.N.; et al. Essential Amino Acid Supplementation Mitigates Muscle Atrophy After Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial. JB JS Open Access 2018, 3, e0006. [Google Scholar] [CrossRef]
- Henriksen, M.G.; Hessov, I.; Dela, F.; Hansen, H.V.; Haraldsted, V.; Rodt, S.A. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol. Scand. 2003, 47, 191–199. [Google Scholar] [CrossRef]
- Singh, J.A.; Lewallen, D.G. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J. Am. Geriatr. Soc. 2010, 58, 2387–2393. [Google Scholar] [CrossRef] [Green Version]
- Smith-Ryan, A.E.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Blue, M.N.M. Nutritional Considerations and Strategies to Facilitate Injury Recovery and Rehabilitation. J. Athl. Train. 2020, 55, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.C.; Phillips, S.M.; Wainwright, T.W. What is the role of nutritional supplements in support of total hip replacement and total knee replacement surgeries? A systematic review. Nutrients 2018, 10, 820. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, R.S.; Tufts, C.W.; De Pinto, D.G.; Chen, J.; Altshuler, J.R.; Serdiuk, A.; Cohen, J.B.; Patel, S.Y. How sweet is this? A review and evaluation of preoperative carbohydrate loading in the enhanced recovery after surgery model. Nutr. Clin. Pract. 2020, 35, 246–253. [Google Scholar] [CrossRef]
- Nygren, J. The metabolic effects of fasting and surgery. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 429–438. [Google Scholar] [CrossRef]
- Svanfeldt, M.; Thorell, A.; Hausel, J.; Soop, M.; Rooyackers, O.; Nygren, J.; Ljungqvist, O. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br. J. Surg. 2007, 94, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.L.; Magnusson, I.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Landau, B.R.; Wahren, J.; Chandramouli, V.; Schumann, W.C.; Ekberg, K.; Kalhan, S.C. Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Invest. 1996, 98, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.M.; Chiolero, R.; Revelly, J.P.; Cayeux, C.; Schneiter, P.; Jequier, E.; Chen, T.; Tappy, L. Effects of enteral carbohydrates on de novo lipogenesis in critically ill patients. Am. J. Clin. Nutr. 2000, 72, 940–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soop, M.; Carlson, G.L.; Hopkinson, J.; Clarke, S.; Thorell, A.; Nygren, J.; Ljungqvist, O. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br. J. Surg. 2004, 91, 1138–1145. [Google Scholar] [CrossRef]
- Svanfeldt, M.; Thorell, A.; Nygren, J.; Ljungqvist, O. Postoperative parenteral nutrition while proactively minimizing insulin resistance. Nutrition 2006, 22, 457–464. [Google Scholar] [CrossRef]
- Phillips, S.M.; Paddon-Jones, D.; Layman, D.K. Optimizing Adult Protein Intake During Catabolic Health Conditions. Adv. Nutr. 2020, 11, S1058–S1069. [Google Scholar] [CrossRef]
- Paddon-Jones, D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J. Nutr. 2006, 136, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Johannsen, M.; Jorgensen, J.O.; Richelsen, B.; Jessen, N.; Moller, N. Amino acid supplementation is anabolic during the acute phase of endotoxin-induced inflammation: A human randomized crossover trial. Clin. Nutr. 2016, 35, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Azhar, G.; Wei, J.Y.; Schutzler, S.E.; Coker, K.; Gibson, R.V.; Kirby, M.F.; Ferrando, A.A.; Wolfe, R.R. Daily consumption of a spe-cially formulated essential amino acid-based dietary supplement improves physical performance in older adults with low physi-cal functioning. J. Gerontol. A Biol. Sci. Med. Sci. 2021. [CrossRef]
- Baldissarro, E.; Aquilani, R.; Boschi, F.; Baiardi, P.; Iadarola, P.; Fumagalli, M.; Pasini, E.; Verri, M.; Dossena, M.; Gambino, A.; et al. The Hip Functional Retrieval after Elective Surgery May Be Enhanced by Supplemented Essential Amino Acids. BioMed Res. Int. 2016, 2016, 9318329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrando, A.; Bamman, M.; Schutzler, S.; Spencer, H.; Dawson, A.; Evans, R.; Wolfe, R. Increased nitrogen intake following hip arthroplasty expedites muscle strength recovery. J. Aging Res. Clin. Pract. 2013, 2, 369–375. [Google Scholar]
- Aquilani, R.; Zuccarelli, G.C.; Condino, A.M.; Catani, M.; Rutili, C.; Del Vecchio, C.; Pisano, P.; Verri, M.; Iadarola, P.; Viglio, S.; et al. Despite Inflammation, Supplemented Essential Amino Acids May Improve Circulating Levels of Albumin and Haemoglobin in Patients after Hip Fractures. Nutrients 2017, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Eddleston, J.; McCairn, A.; Dowling, S.; McWilliams, D.; Coughlan, E.; Griffiths, R.D. Improving rehabilitation after critical illness through outpatient physiotherapy classes and essential amino acid supplement: A randomized controlled trial. J. Crit. Care 2015, 30, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Zuccarelli Ginetto, C.; Rutili, C.; Pisano, P.; Pasini, E.; Baldissarro, E.; Verri, M.; Boschi, F. Supplemented amino acids may enhance the walking recovery of elderly subjects after hip fracture surgery. Aging Clin. Exp. Res. 2019, 31, 157–160. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Aarsland, A.; Cree, M.G.; Wolfe, R.R. Muscle protein synthesis and balance responsiveness to essential amino acids ingestion in the presence of elevated plasma free fatty acid concentrations. J. Clin. Endocrinol. Metab. 2009, 94, 2984–2990. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Sheffield-Moore, M.; Urban, R.J.; Sanford, A.P.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J. Clin. Endocrinol. Metab. 2004, 89, 4351–4358. [Google Scholar] [CrossRef]
- Fitts, R.H.; Romatowski, J.G.; Peters, J.R.; Paddon-Jones, D.; Wolfe, R.R.; Ferrando, A.A. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am. J. Physiol. Cell Physiol. 2007, 293, C313–C320. [Google Scholar] [CrossRef]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeters, P.B.; Shenkin, A.; Sobotka, L.; Soeters, M.R.; de Leeuw, P.W.; Wolfe, R.R. The anabolic role of the Warburg, Cori-cycle and Crabtree effects in health and disease. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Baum, J.I.; Starck, C.; Moughan, P.J. Factors contributing to the selection of dietary protein food sources. Clin. Nutr. 2018, 37, 130–138. [Google Scholar] [CrossRef]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Morton, J.P.; van Loon, L.J. Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. Eur. J. Sport Sci. 2015, 15, 53–62. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Church, D.D.; Schutzler, S.E.; Azhar, G.; Kim, I.Y.; Ferrando, A.A.; Wolfe, R.R. Metabolic evaluation of the Dietary Guideline’s ounce equivalents of protein food sources in young adults: A randomized controlled trial. J. Nutr. 2021, in press. [Google Scholar] [CrossRef]
- Vliet, S.V.; Beals, J.W.; Martinez, I.G.; Skinner, S.K.; Burd, N.A. Achieving Optimal Post-Exercise Muscle Protein Remodeling in Physically Active Adults through Whole Food Consumption. Nutrients 2018, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Hevia-Larraín, V.; Gualano, B.; Longobardi, I.; Gil, S.; Fernandes, A.L.; Costa, L.A.; Pereira, R.M.; Artioli, G.G.; Phillips, S.M.; Roschel, H. High-Protein Plant-Based Diet Versus a Protein-Matched Omnivorous Diet to Support Resistance Training Adaptations: A Comparison Between Habitual Vegans and Omnivores. Sports Med. 2021, 1–14. [Google Scholar] [CrossRef]
- Van Vliet, S.; Provenza, F.D.; Kronberg, S.L. Health-Promoting Compounds are Higher in Grass-Fed Meat and Milk. Front. Sustain. Food Syst. 2020, 4, 299. [Google Scholar]
- Baur, D.A.; Saunders, M.J. Carbohydrate supplementation: A critical review of recent innovations. Eur. J. Appl. Physiol. 2021, 121, 23–66. [Google Scholar] [CrossRef]
- Volek, J.S. SuperStarch: A Technological Breakthrough in Sports Nutrition Innovation; Generation UCAN: Woodbridge, CT, USA, 2019. [Google Scholar]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Trommelen, J.; Betz, M.W.; van Loon, L.J. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.; LJ, V.A.N.L. Protein ingestion before sleep improves postexercise overnight recovery. Med. Sci. Sports Exerc. 2012, 44, 1560–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, J.L.; Keerati, U.R.M.; Yin, H.; Daoust, J.; Nonnotte, E.; Quinquis, L.; St-Denis, T.; Bolster, D.R. Differential Responses of Blood Essential Amino Acid Levels Following Ingestion of High-Quality Plant-Based Protein Blends Compared to Whey Protein-A Double-Blind Randomized, Cross-Over, Clinical Trial. Nutrients 2019, 11, 2987. [Google Scholar] [CrossRef] [Green Version]
- Ali Abdelhamid, Y.; Chapman, M.; Deane, A. Peri-operative nutrition. Anaesthesia 2016, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021, 1–10. [Google Scholar] [CrossRef]
- Gualano, A.B.; Bozza, T.; Lopes De Campos, P.; Roschel, H.; Dos Santos Costa, A.; Luiz Marquezi, M.; Benatti, F.; Herbert Lancha Junior, A. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fit. 2011, 51, 82–88. [Google Scholar]
- Paddon-Jones, D.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E761–E767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyskens, J.B.; Foote, D.M.; Bigot, N.J.; Strycker, L.A.; Smolkowski, K.; Kirkpatrick, T.K.; Lantz, B.A.; Shah, S.N.; Mohler, C.G.; Jewett, B.A.; et al. Cellular and morphological changes with EAA supplementation before and after total knee arthroplasty. J. Appl. Physiol. 2019, 127, 531–545. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. 2015, 45 (Suppl. 1), S93–S104. [Google Scholar] [CrossRef] [Green Version]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, E.E.; Pasiakos, S.M.; Fussell, M.A.; Rodriguez, N.R. Skeletal Muscle Disuse Atrophy and the Rehabilitative Role of Protein in Recovery from Musculoskeletal Injury. Adv. Nutr. 2020, 11, 989–1001. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirsch, K.R.; Wolfe, R.R.; Ferrando, A.A. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients 2021, 13, 1675. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051675
Hirsch KR, Wolfe RR, Ferrando AA. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients. 2021; 13(5):1675. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051675
Chicago/Turabian StyleHirsch, Katie R., Robert R. Wolfe, and Arny A. Ferrando. 2021. "Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery" Nutrients 13, no. 5: 1675. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051675