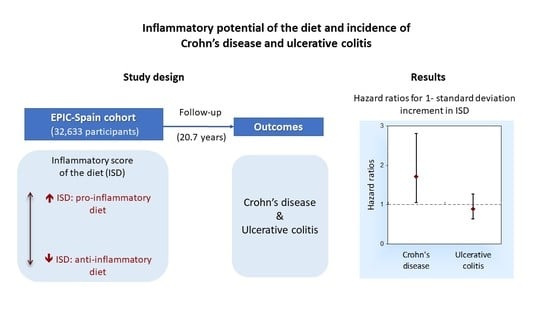
Inflammatory Potential of the Diet and Incidence of Crohn’s Disease and Ulcerative Colitis in the EPIC-Spain Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Exposure Measurements: Diet and Inflammatory Score of the Diet -ISD-
2.3. Assessment of Other Covariates
2.4. Outcome Measurements: Crohn’s Disease and Ulcerative Colitis
2.5. Statistical Analysis
3. Results
3.1. ISD and Risk of Crohn Disease
3.2. ISD and Risk of Ulcerative Colitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamabe, K.; Liebert, R.; Flores, N.; Pashos, C.L. Health-related quality of life outcomes and economic burden of inflammatory bowel disease in Japan. Clinicoecon. Outcomes Res. 2019, 11, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowski, M.; Witkowski, M.; Gagliani, N.; Huber, S. Recipe for IBD: Can we use food to control inflammatory bowel disease? Semin. Immunopathol. 2018, 40, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.; Tjonneland, A.; Olsen, A.; Overvad, K.; Bergmann, M.M.; Boeing, H.; Nagel, G.; Linseisen, J.; Hallmans, G.; Danielsson, Å.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar]
- Costea, I.; Mack, D.R.; Lemaitre, R.N.; Israel, D.; Marcil, V.; Ahmad, A.; Amre, D.K. Interactions between the dietary polyunsaturated fatty acid ratio and genetic factors determine susceptibility to pediatric Crohn’s disease. Gastroenterology 2014, 146, 929–931. [Google Scholar] [CrossRef] [Green Version]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary fiber intake reduces risk of inflammatory bowel disease: Result from a meta-analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, S.; Levy, E.; Mack, D.; Israel, D.; Lambrette, P.; Ghadirian, P.; Deslandres, C.; Morgan, K.; Seidman, E.G.; Amre, D.K. Dietary patterns and risk for Crohn’s disease in children. Inflamm. Bowel Dis. 2008, 14, 367–373. [Google Scholar] [CrossRef]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef]
- Lo, C.-H.; Lochhead, P.; Khalili, H.; Song, M.; Tabung, F.K.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Dietary Inflammatory Potential and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2020, 159, 873–883.e1. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rashvand, S.; Rashidkhani, B.; Hekmatdoost, A. Inflammatory Potential of Diet and Risk of Ulcerative Colitis in a Case-Control Study from Iran. Nutr. Cancer 2016, 68, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Khademi, Z.; Saneei, P.; Hassanzadeh-Keshteli, A.; Daghaghzadeh, H.; Tavakkoli, H.; Adibi, P.; Esmaillzadeh, A. Association Between Inflammatory Potential of the Diet and Ulcerative Colitis: A Case-Control Study. Front. Nutr. 2021, 7, 602090. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J. Dietary Inflammatory Index and Biomarkers of Lipoprotein Metabolism, Inflammation and Glucose Homeostasis in Adults. Nutrients 2018, 10, 1033. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Agudo, A.; Masegú, R.; Bonet, C.; Jakszyn, P.; Quirós, J.R.; Ardanaz, E.; Moreno-Iribas, C.; Barricarte, A.; Amiano, P.; Arriola, L.; et al. Inflammatory potential of the diet and mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Mol. Nutr. Food Res. 2017, 61, 1600649. [Google Scholar] [CrossRef] [PubMed]
- Agudo, A.; Cayssials, V.; Bonet, C.; Tjonneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Affret, A.; Fagherazzi, G.; Katzke, V.; Schubel, R.; et al. Inflammatory potential of the diet and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2018, 107, 607–616. [Google Scholar] [CrossRef]
- Jakszyn, P.; Cayssials, V.; Buckland, G.; Perez-Cornago, A.; Weiderpass, E.; Boeing, H.; Bergmann, M.M.; Vulcan, A.; Ohlsson, B.; Masala, G.; et al. Inflammatory potential of the diet and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2020, 147, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Benavente, Y.; Saez, M.; Agudo, A.; Jakszyn, P.; Naudin, S.; Hosnijeh, F.S.; Gunter, M.; Huybrechts, I.; Ferrari, P.; et al. Inflammatory potential of diet and risk of lymphoma in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Nutr. 2020, 59, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, M.E.; Akinyemiju, T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef] [Green Version]
- Tyrovolas, S.; Koyanagi, A.; Kotsakis, G.A.; Panagiotakos, D.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Haro, J.M. Dietary inflammatory potential is linked to cardiovascular disease risk burden in the US adult population. Int. J. Cardiol. 2017, 240, 409–413. [Google Scholar] [CrossRef]
- Graffouillere, L.; Deschasaux, M.; Mariotti, F.; Neufcourt, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Latino-Martel, P.; Hercberg, S.; Galan, P.; et al. Prospective association between the Dietary Inflammatory Index and mortality: Modulation by antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riboli, E.; Hunt, K.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2003, 5, 1113–1124. [Google Scholar] [CrossRef]
- González, C.A.; Navarro, C.; Martínez, C.; Quirós, J.R.; Dorronsoro, M.; Barricarte, A.; Tormo, M.J.; Agudo, A.; Chirlaque, M.D.; Amiano, P.; et al. The European prospective investigation about cancer and nutrition (EPIC) (article in Spanish). Rev. Esp. Salud Publica 2004, 78, 167–176. [Google Scholar] [CrossRef] [Green Version]
- EPIC Group of Spain. Relative validity and reproducibility of a diet history questionnaire in Spain. II. Nutrients. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S100–S109. [Google Scholar]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.T.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Møller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhof, A.; Woodward, M.; Doering, A.; Helbecque, N.; Loewel, H.; Amouyel, P.; Lowe, G.D.O.; Koenig, W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: Results from three MONICA samples (Augsburg, Glasgow, Lille). Eur. Heart J. 2004, 25, 2092–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, S.; Mehta, G.; Moore, K.; Britton, A. Ten-year alcohol consumption typologies and trajectories of C-reactive protein, interleukin-6 and interleukin-1 receptor antagonist over the following 12 years: A prospective cohort study. J. Intern. Med. 2017, 281, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Peters, T.; Brage, S.; Westgate, K.; Franks, P.W.; Gradmark, A.; Diaz, M.J.T.; Huerta, J.M.; Bendinelli, B.; Vigl, M.; Boeing, H.; et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur. J. Epidemiol. 2012, 27, 15–25. [Google Scholar] [PubMed] [Green Version]
- Vagianos, K.; Shafer, L.A.; Witges, K.; Targownik, L.E.; Haviva, C.; Graff, L.A.; Sexton, K.A.; Lix, L.M.; Sargent, M.; Bernstein, C.N. Association between Change in Inflammatory Aspects of Diet and Change in IBD-related Inflammation and Symptoms over 1 Year: The Manitoba Living with IBD Study. Inflamm. Bowel Dis. 2021, 27, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Moslehi, N.; Morshedzadeh, N.; Shivappa, N.; Hébert, J.R.; Farsi, F.; Daryani, N.E. Does the inflammatory potential of diet affect disease activity in patients with inflammatory bowel disease? Nutr. J. 2019, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350. [Google Scholar] [CrossRef]
- Dias, J.A.; Wirfält, E.; Drake, I.; Gullberg, B.; Hedblad, B.; Persson, M.; Engström, G.; Nilsson, J.; Schiopu, A.; Fredrikson, G.N.; et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis 2015, 238, 38–44. [Google Scholar] [CrossRef]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G.; MOLI-SANI Study Investigators. Mediterranean diet, dietary polyphenols and low grade inflammation: Results from the MOLI-SANI study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: The association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Sun, K.; Wu, Y.; Yang, Y.; Tso, P.; Wu, Z. Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease. Front. Immunol. 2017, 8, 942. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]

| All Participants | Inflammatory Score of the Diet Tertiles a | p-Value b | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Total cohort, n | 32,663 | 10,888 | 10,888 | 10,887 | |
| Sex, n (%) | <0.001 | ||||
| Women | 20,168 (61.7) | 4513 (41.4) | 6796 (62.4) | 8859 (81.37) | |
| Men | 12,495 (38.3) | 6375 (58.5) | 4092 (37.6) | 2028 (18.6) | |
| Age at recruitment [years], n (%) | <0.001 | ||||
| <40 | 4171 (12.8) | 968 (8.9) | 1393 (12.8) | 1810 (16.6) | |
| 40–<50 | 13,655 (41.8) | 4785 (43.9) | 4627(42.5) | 4243 (39.0) | |
| 50–<60 | 10,665 (32.6) | 3886 (35.7) | 3566 (32.8) | 3213 (29.5) | |
| ≥60 | 4172 (12.8) | 1249 (11.5) | 1302 (12.0) | 1621 (14.9) | |
| Body mass index [kg/m2], mean (SD) | 28.3 (4.3) | 28.2 (4.0) | 28.2 (4.3) | 28.5 (4.6) | <0.001 |
| Educational level, n (%) | <0.001 | ||||
| No formal education | 11,965 (36.6) | 3460 (31.8) | 3858 (35.4) | 4647 (42.7) | |
| Primary school | 12,183 (37.3) | 4199 (38.6) | 4139 (38.0) | 3845 (35.3) | |
| Technical or professional training | 2637 (8.1) | 1057 (9.7) | 906 (8.3) | 674 (6.2) | |
| Secondary school | 1921 (5.9) | 687 (6.3) | 647 (5.9) | 587 (5.4) | |
| University degree | 3750 (11.5) | 1436 (13.2) | 1255 (11.5) | 1059 (9.7) | |
| Not specified | 207 (0.63) | 49 (0.4) | 83 (0.7) | 75 (0.7) | |
| Physical activity, n (%) | <0.001 | ||||
| Inactive | 13,680 (41.9) | 4052 (37.22) | 4495 (41.3) | 5133 (47.1) | |
| Moderately inactive | 9468 (29.0) | 3017 (27.7) | 3228(29.6) | 3223(26.6) | |
| Moderately active | 5881 (18.0) | 2164 (19.9) | 2004 (18.4) | 1713 (15.7) | |
| Active | 3634 (11.1) | 1655 (15.2) | 1161 (10.7) | 818 (7.5) | |
| Smoking status, n (%) | <0.001 | ||||
| Never | 18,266 (55.9) | 5298 (48.7) | 6089 (55.9) | 6879 (63.2) | |
| Former | 5666 (17.3) | 2460 (22.6) | 1905 (17.5) | 1301 (11.9) | |
| Current | 8716 (26.7) | 3126 (28.7) | 2890 (26.5) | 2700 (24.8) | |
| Unknown | 15 (0.1) | 4 (0.0) | 4 (0.0) | 7 (0.1) | |
| Energy intake [kcal/day], mean (SD) | 2174 (704) | 2674 (699) | 2143 (561) | 1705 (461) | <0.001 |
| ISD Tertiles a | 1-SD Increment in ISD (Continuous) | |||||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | P-Trend | HR (95% CI) | P-Trend | |
| Crohn’s disease | ||||||
| No. of cases/person-years | 6/225,930 | 13/226,335 | 13/222,283 | |||
| Model 1 b | 0.44 (0.15–1.27) | 1.00 (0.48–2.23) | 1 (ref.) | 0.142 | 1.42 (0.94–2.14) | 0.098 |
| Model 2 c | 0.32 (0.10–1.05) | 0.87 (0.38–2.01) | 1 (ref.) | 0.067 | 1.71 (1.05–2.80) | 0.031 |
| Ulcerative colitis | ||||||
| No. of cases/person-years | 23/225,930 | 19/226,335 | 15/222,283 | |||
| Model 1 b | 1.48 (0.71–3.06) | 1.27 (0.63–2.55) | 1 (ref.) | 0.297 | 0.89 (0.66–1.19) | 0.436 |
| Model 2 c | 1.49 (0.66–3.34) | 1.27 (0.62–2.60) | 1 (ref.) | 0.340 | 0.89 (0.63–1.26) | 0.515 |
| ISD Tertiles a | 1-SD Increment in ISD (Continuous) | |||||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | P-Trend | HR (95% CI) | P-Trend | |
| Crohn’s disease | ||||||
| Model 2 + smoking + physical activity | 0.35 (0.10–1.15) | 0.91 (0.39–2.10) | 1 (ref.) | 0.095 | 1.64 (1.00–2.67) | 0.050 |
| Model 2 after excluding the first 2 years of follow-up b | 0.36 (0.10–1.13) | 0.93 (0.40–2.19) | 1 (ref.) | 0.085 | 1.72 (1.05–2.83) | 0.032 |
| Ulcerative colitis | ||||||
| Model 2 + smoking + physical activity | 1.51 (0.67–3.42) | 1.28 (0.62–2.62) | 1 (ref.) | 0.322 | 0.89 (0.63–1.25) | 0.498 |
| Model 2 after excluding the first 2 years of follow-up b | 1.41 (0.62–3.22) | 1.07 (0.51–2.26) | 1 (ref.) | 0.398 | 0.92 (0.65–1.32) | 0.667 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara, M.; Salamanca-Fernández, E.; Miqueleiz, E.; Gavrila, D.; Amiano, P.; Bonet, C.; Rodríguez-Barranco, M.; Huerta, J.M.; Bujanda, L.; Sánchez, M.J.; et al. Inflammatory Potential of the Diet and Incidence of Crohn’s Disease and Ulcerative Colitis in the EPIC-Spain Cohort. Nutrients 2021, 13, 2201. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072201
Guevara M, Salamanca-Fernández E, Miqueleiz E, Gavrila D, Amiano P, Bonet C, Rodríguez-Barranco M, Huerta JM, Bujanda L, Sánchez MJ, et al. Inflammatory Potential of the Diet and Incidence of Crohn’s Disease and Ulcerative Colitis in the EPIC-Spain Cohort. Nutrients. 2021; 13(7):2201. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072201
Chicago/Turabian StyleGuevara, Marcela, Elena Salamanca-Fernández, Estrella Miqueleiz, Diana Gavrila, Pilar Amiano, Catalina Bonet, Miguel Rodríguez-Barranco, José María Huerta, Luis Bujanda, María José Sánchez, and et al. 2021. "Inflammatory Potential of the Diet and Incidence of Crohn’s Disease and Ulcerative Colitis in the EPIC-Spain Cohort" Nutrients 13, no. 7: 2201. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072201