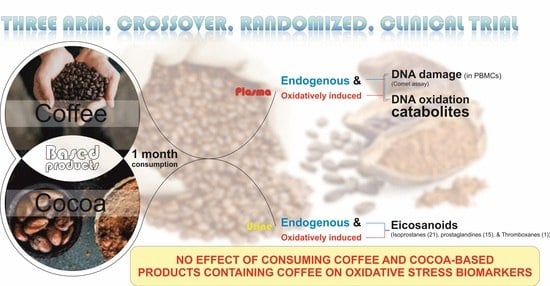
Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Products
2.2. Participants
2.3. Study Design and Protocol
2.4. Total Antioxidant Capacity of the Diet
2.5. Analysis of Markers of DNA Damage by Comet Assay
2.5.1. Chemicals and Reagents
2.5.2. Sample Preparation and Analysis
2.6. Analysis of DNA Oxidation Catabolites
2.6.1. Chemicals and Reagents
2.6.2. Extraction and Processing of the Samples
2.6.3. Analysis of DNA Oxidation Catabolites
2.7. Analysis of Lipid Oxidation Catabolites (Oxylipins)
2.7.1. Chemicals and Reagents
2.7.2. Extraction and Processing of the Samples
2.7.3. Analysis of the Lipid Oxidation Catabolites
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Markers of DNA Damage
3.2.1. Endogenous and Oxidatively Induced DNA Damage
3.2.2. DNA Oxidation Catabolites
3.3. Lipid Peroxidation Products (Oxylipins)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2020; pp. 65–96. ISBN 9780128204702. [Google Scholar]
- Angelino, D.; Tassotti, M.; Brighenti, F.; Del Rio, D.; Mena, P. Niacin, alkaloids and (poly) phenolic compounds in the most widespread Italian capsule-brewed coffees. Sci. Rep. 2018, 8, 17874. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Kim, H.-T.; Jeong, I.-H.; Hong, S.-R.; Oh, M.-S.; Yoon, M.-H.; Shim, J.-H.; Jeong, J.H.; Abd El-Aty, A.M. Contents of chlorogenic acids and caffeine in various coffee-related products. J. Adv. Res. 2019, 17, 85–94. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [Green Version]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.-M.; Zuluaga, N.; Gil-Izquierdo, Á.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic. Biol. Med. 2020, 160, 604–617. [Google Scholar] [CrossRef] [PubMed]
- León, D.; Medina, S.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Ferreres, F.; Gil-Izquierdo, A. Anti-inflammatory Activity of Coffee. In Coffee; Royal Society of Chemistry: Cambridge, MA, USA, 2019; Chapter 3; pp. 57–74. ISBN 978-1-78801-497-7. [Google Scholar]
- Lafay, S.; Gil-Izquierdo, A. Effect of Coffee on Weight Management. In Coffee; Royal Society of Chemistry: Cambridge, MA, USA, 2019; Chapter 12; pp. 265–285. ISBN 978-1-78801-497-7. [Google Scholar]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, j5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscemi, S.; Marventano, S.; Antoci, M.; Cagnetti, A.; Castorina, G.; Galvano, F.; Marranzano, M.; Mistretta, A. Coffee and metabolic impairment: An updated review of epidemiological studies. NFS J. 2016, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Del Bo, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly) Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Marino, M.; Martini, D.; Tucci, M.; Ciappellano, S.; Riso, P.; Porrini, M. Overview of Human Intervention Studies Evaluating the Impact of the Mediterranean Diet on Markers of DNA Damage. Nutrients 2019, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Riso, P.; Martini, D.; Møller, P.; Loft, S.; Bonacina, G.; Moro, M.; Porrini, M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 2010, 25, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinkellner, H.; Hoelzl, C.; Uhl, M.; Cavin, C.; Haidinger, G.; Gsur, A.; Schmid, R.; Kundi, M.; Bichler, J.; Knasmüller, S. Coffee consumption induces GSTP in plasma and protects lymphocytes against (±)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: Results of controlled human intervention trials. Mutat. Res. Mol. Mech. Mutagen. 2005, 591, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Bichler, J.; Cavin, C.; Simic, T.; Chakraborty, A.; Ferk, F.; Hoelzl, C.; Schulte-Hermann, R.; Kundi, M.; Haidinger, G.; Angelis, K.; et al. Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) induced DNA-damage: Results of an experimental study with human volunteers. Food Chem. Toxicol. 2007, 45, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, S.; Hatzold, T.; El Yamani, N.; Stavro, P.M.; Lorenzo, Y.; Dusinska, M.; Reus, A.; Pasman, W.; Collins, A. Coffee and oxidative stress: A human intervention study. Eur. J. Nutr. 2018, 57, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hoelzl, C.; Knasmüller, S.; Wagner, K.-H.; Elbling, L.; Huber, W.; Kager, N.; Ferk, F.; Ehrlich, V.; Nersesyan, A.; Neubauer, O.; et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010, 54, 1722–1733. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Yukawa, G.S.; Mune, M.; Otani, H.; Tone, Y.; Liang, X.-M.; Iwahashi, H.; Sakamoto, W. Effects of Coffee Consumption on Oxidative Susceptibility of Low-Density Lipoproteins and Serum Lipid Levels in Humans. Biochemistry 2004, 69, 70–74. [Google Scholar] [CrossRef]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Alfthan, G.; Virtanen, J.K.; Rissanen, T.H.; Happonen, P.; Nyyssönen, K.; Kaikkonen, J.; Salonen, R.; et al. The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: A clinical trial. Free Radic. Biol. Med. 2005, 38, 527–534. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Martini, D.; Rosi, A.; Brighenti, F.; Del Rio, D. The Pocket-4-Life project, bioavailability and beneficial properties of the bioactive compounds of espresso coffee and cocoa-based confectionery containing coffee: Study protocol for a randomized cross-over trial. Trials 2017, 18, 527. [Google Scholar] [CrossRef]
- Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Dei Cas, A.; Bonadonna, R.; Brighenti, F.; Del Rio, D.; Mena, P. Absorption, Pharmacokinetics, and Urinary Excretion of Pyridines After Consumption of Coffee and Cocoa-Based Products Containing Coffee in a Repeated Dose, Crossover Human Intervention Study. Mol. Nutr. Food Res. 2020, 64, 2000489. [Google Scholar] [CrossRef]
- Favari, C.; Righetti, L.; Tassotti, M.; Gethings, L.A.; Martini, D.; Rosi, A.; Antonini, M.; Rubert, J.; Manach, C.; Dei Cas, A.; et al. Metabolomic Changes after Coffee Consumption: New Paths on the Block. Mol. Nutr. Food Res. 2021, 65, 2000875. [Google Scholar] [CrossRef]
- Martini, D.; Rosi, A.; Tassotti, M.; Antonini, M.; Dall’Asta, M.; Bresciani, L.; Fantuzzi, F.; Spigoni, V.; Domínguez-Perles, R.; Angelino, D.; et al. Effect of coffee and cocoa-based confectionery containing coffee on markers of cardiometabolic health: Results from the pocket-4-life project. Eur. J. Nutr. 2020, 60, 1453–1463. [Google Scholar] [CrossRef]
- Mehrabani, S.; Arab, A.; Mohammadi, H.; Amani, R. The effect of cocoa consumption on markers of oxidative stress: A systematic review and meta-analysis of interventional studies. Complement. Ther. Med. 2020, 48, 102240. [Google Scholar] [CrossRef]
- Pellegrini, N.; Salvatore, S.; Valtueña, S.; Bedogni, G.; Porrini, M.; Pala, V.; Del Rio, D.; Sieri, S.; Miglio, C.; Krogh, V.; et al. Development and Validation of a Food Frequency Questionnaire for the Assessment of Dietary Total Antioxidant Capacity. J. Nutr. 2007, 137, 93–98. [Google Scholar] [CrossRef]
- Møller, P. The comet assay: Ready for 30 more years. Mutagenesis 2018, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Pereira, C.C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for describing comet assay procedures and results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Fracassetti, D.; Lanti, C.; Porrini, M.; Riso, P. Comparison of DNA damage by the comet assay in fresh versus cryopreserved peripheral blood mononuclear cells obtained following dietary intervention. Mutagenesis 2015, 30, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaño, D.; Vilaplana, C.; Medina, S.; Cejuela-Anta, R.; Martínez-Sanz, J.M.; Gil, P.; Genieser, H.-G.; Ferreres, F.; Gil-Izquierdo, A. Effect of elite physical exercise by triathletes on seven catabolites of DNA oxidation. Free Radic. Res. 2015, 49, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Genieser, H.-G.; Ferreres, F.; Gil-Izquierdo, Á. Melatonin and hydroxytyrosol-rich wines influence the generation of DNA oxidation catabolites linked to mutagenesis after the ingestion of three types of wine by healthy volunteers. Food Funct. 2016, 7, 4781–4796. [Google Scholar] [CrossRef]
- García-Flores, L.A.; Medina, S.; Cejuela-Anta, R.; Martínez-Sanz, J.M.; Abellán, Á.; Genieser, H.-G.; Ferreres, F.; Gil-Izquierdo, Á. DNA catabolites in triathletes: Effects of supplementation with an aronia–citrus juice (polyphenols-rich juice). Food Funct. 2016, 7, 2084–2093. [Google Scholar] [CrossRef] [Green Version]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.-M. Stereocontrolled Access to Isoprostanes via a Bicyclo [3.3.0] octene Framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, Y.; Oger, C.; Guy, A.; Durand, T.; Galano, J.-M. Total Synthesis of 15-D 2t-and 15-epi-15-E 2t -Isoprostanes. J. Org. Chem. 2010, 75, 2411–2414. [Google Scholar] [CrossRef]
- Guy, A.; Oger, C.; Heppekausen, J.; Signorini, C.; De Felice, C.; Fürstner, A.; Durand, T.; Galano, J.-M. Oxygenated Metabolites of n-3 Polyunsaturated Fatty Acids as Potential Oxidative Stress Biomarkers: Total Synthesis of 8-F 3t-IsoP, 10-F 4t-NeuroP and [D 4]-10-F 4t-NeuroP. Chem. A Eur. J. 2014, 20, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; De Miguel-Elízaga, I.; Oger, C.; Galano, J.-M.; Durand, T.; Martínez-Villanueva, M.; Castillo, M.L.G.-D.; Villegas-Martínez, I.; Ferreres, F.; Martínez-Hernández, P.; et al. Dihomo-isoprostanes—nonenzymatic metabolites of AdA—are higher in epileptic patients compared to healthy individuals by a new ultrahigh pressure liquid chromatography–triple quadrupole–tandem mass spectrometry method. Free Radic. Biol. Med. 2015, 79, 154–163. [Google Scholar] [CrossRef]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.-M.; Durand, T.; Solana, A.; et al. Effect of the dietary intake of melatonin- and hydroxytyrosol-rich wines by healthy female volunteers on the systemic lipidomic-related oxylipins. Food Funct. 2017, 8, 3745–3757. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Domínguez-Perles, R.; Gil, J.I.; Ferreres, F.; García-Viguera, C.; Martínez-Sanz, J.M.; Gil-Izquierdo, A. A ultra-pressure liquid chromatography/triple quadrupole tandem mass spectrometry method for the analysis of 13 eicosanoids in human urine and quantitative 24 hour values in healthy volunteers in a controlled constant diet. Rapid Commun. Mass Spectrom. 2012, 26, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Domínguez-Perles, R.; Cejuela-Anta, R.; Villaño, D.; Martínez-Sanz, J.M.; Gil, P.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, A. Assessment of oxidative stress markers and prostaglandins after chronic training of triathletes. Prostaglandins Other Lipid Mediat. 2012. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, Á. The intake of broccoli sprouts modulates the inflammatory and vascular prostanoids but not the oxidative stress-related isoprostanes in healthy humans. Food Chem. 2015, 173, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.-M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Martini, D.; Rossi, S.; Biasini, B.; Zavaroni, I.; Bedogni, G.; Musci, M.; Pruneti, C.; Passeri, G.; Ventura, M.; Di Nuzzo, S.; et al. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; De Las Heras-Gómez, I.; Casas-Pina, T.; Bultel-Poncé, V.; Galano, J.-M.; Durand, T.; Martínez-Hernández, P.; Ferreres, F.; Jimeno, L.; Llorente, S.; et al. Urinary oxylipin signature as biomarkers to monitor the allograft function during the first six months post-renal transplantation. Free Radic. Biol. Med. 2020, 146, 340–349. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, L.A.; Medina, S.; Gómez, C.; Wheelock, C.E.; Cejuela, R.; Martínez-Sanz, J.M.; Oger, C.; Galano, J.-M.; Durand, T.; Hernández-Sáez, Á.; et al. Aronia–citrus juice (polyphenol-rich juice) intake and elite triathlon training: A lipidomic approach using representative oxylipins in urine. Food Funct. 2018, 9, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senn, S. Cross-over Trials in Clinical Research, 2nd ed.; Senn, S.S., Barnett, V., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002; ISBN 0471496533. [Google Scholar]
- Morze, J.; Schwedhelm, C.; Bencic, A.; Hoffmann, G.; Boeing, H.; Przybylowicz, K.; Schwingshackl, L. Chocolate and risk of chronic disease: A systematic review and dose-response meta-analysis. Eur. J. Nutr. 2020, 59, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo, C.; Riso, P.; et al. Application of the comet assay in human biomonitoring: An hCOMET perspective. Mutat. Res. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef]
- Mišík, M.; Hoelzl, C.; Wagner, K.-H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. Mol. Mech. Mutagen. 2010, 692, 42–48. [Google Scholar] [CrossRef]
- Cardin, R.; Piciocchi, M.; Martines, D.; Scribano, L.; Petracco, M.; Farinati, F. Effects of coffee consumption in chronic hepatitis C: A randomized controlled trial. Dig. Liver Dis. 2013, 45, 499–504. [Google Scholar] [CrossRef]
- Pahlke, G.; Attakpah, E.; Aichinger, G.; Ahlberg, K.; Hochkogler, C.M.; Schweiger, K.; Schipp, D.; Somoza, V.; Marko, D. Dark coffee consumption protects human blood cells from spontaneous DNA damage. J. Funct. Foods 2019, 55, 285–295. [Google Scholar] [CrossRef]
- Schipp, D.; Tulinska, J.; Sustrova, M.; Liskova, A.; Spustova, V.; Mikusova, M.L.; Krivosikova, Z.; Rausova, K.; Collins, A.; Vebraite, V.; et al. Consumption of a dark roast coffee blend reduces DNA damage in humans: Results from a 4-week randomised controlled study. Eur. J. Nutr. 2019, 58, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.-P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797. [Google Scholar] [CrossRef]
- Bakuradze, T.; Parra, G.A.M.; Riedel, A.; Somoza, V.; Lang, R.; Dieminger, N.; Hofmann, T.; Winkler, S.; Hassmann, U.; Marko, D.; et al. Four-week coffee consumption affects energy intake, satiety regulation, body fat, and protects DNA integrity. Food Res. Int. 2014, 63, 420–427. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Eisenbrand, G.; Schipp, D.; Galan, J.; Richling, E. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: A randomized controlled trial. Eur. J. Nutr. 2015, 54, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: Results of a short-term repeated uptake intervention study. Mol. Nutr. Food Res. 2016, 60, 682–686. [Google Scholar] [CrossRef]
- Hoffmann, H.; Hogel, J.; Speit, G. The effect of smoking on DNA effects in the comet assay: A meta-analysis. Mutagenesis 2005, 20, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Parzefall, W.; Knasmüller, S. Single cell gel electrophoresis assay: A new technique for human biomonitoring studies. Mutat. Res. 2000, 463, 13–31. [Google Scholar] [CrossRef]
- Spadafranca, A.; Conesa, C.M.; Sirini, S.; Testolin, G. Effect of dark chocolate on plasma epicatechin levels, DNA resistance to oxidative stress and total antioxidant activity in healthy subjects. Br. J. Nutr. 2010, 103, 1008–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibero-Baraibar, I.; Azqueta, A.; de Cerain, A.L.; Martinez, J.A.; Zulet, M.A. Assessment of DNA damage using comet assay in middle-aged overweight/obese subjects after following a hypocaloric diet supplemented with cocoa extract. Mutagenesis 2015, 30, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Teekachunhatean, S.; Tosri, N.; Sangdee, C.; Wongpoomchai, R.; Ruangyuttikarn, W.; Puaninta, C.; Srichairatanakool, S. Antioxidant effects after coffee enema or oral coffee consumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012, 31, 643–651. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Trepanowski, J.F.; Farney, T.M. Influence of Acute Coffee Consumption on Postprandial Oxidative Stress. Nutr. Metab. Insights 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Leelarungrayub, D.; Sallepan, M.; Charoenwattana, S. Effects of Acute Caffeinated Coffee Consumption on Energy Utilization Related to Glucose and Lipid Oxidation from Short Submaximal Treadmill Exercise in Sedentary Men. Nutr. Metab. Insights 2011, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, T.A.F.; Monteiro, M.P.; Mendes, T.M.N.; de Oliveira, D.M.; Rogero, M.M.; Benites, C.I.; de Matos Vinagre, C.G.C.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; et al. Medium Light and Medium Roast Paper-Filtered Coffee Increased Antioxidant Capacity in Healthy Volunteers: Results of a Randomized Trial. Plant Foods Hum. Nutr. 2012, 67, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Chiavaroli, L.; González-Sarrías, A.; Bresciani, L.; Palma-Duran, S.A.; Dall’Asta, M.; Deligiannidou, G.-E.; Massaro, M.; Scoditti, E.; Combet, E.; et al. Impact of Foods and Dietary Supplements Containing Hydroxycinnamic Acids on Cardiometabolic Biomarkers: A Systematic Review to Explore Inter-Individual Variability. Nutrients 2019, 11, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari Azad, B.; Daneshzad, E.; Meysamie, A.P.; Koohdani, F. Chronic and acute effects of cocoa products intake on arterial stiffness and platelet count and function: A systematic review and dose-response Meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lv, Y.; Zha, W.; Hong, X.; Luo, Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2159–2170. [Google Scholar] [CrossRef]
| Variable | All Participants (n = 21) |
|---|---|
| Socio-demographic and anthropometric data | |
| Age (y) | 22.9 ± 0.5 |
| Sex (males/females) | 10/11 |
| Smoking habits (smokers/non-smokers) | 8/13 |
| Body weight (kg) | 67.0 ± 2.7 |
| Body mass index (kg/m2) | 22.3 ± 1.7 |
| Habitual coffee consumption (serving/day) | 2.3 ± 0.2 |
| Dietary Total Antioxidants Capacity | |
| TAC (mmol Trolox eq./day) | 8.2 ± 3.3 |
| TRAP (mmol Trolox eq./day) | 11.8 ± 5.6 |
| FRAP (mmol Fe2+ eq./day) | 26.1 ± 11.2 |
| Markers of DNA damage | |
| DNA strand breaks (% DNA in tail, PBS) | 25.4 ± 1.6 |
| H2O2-induced DNA damage (% DNA in tail) | 14.4 ± 1.5 |
| DNA strand breaks (% DNA in tail, EB) | 27.4 ± 2.1 |
| FPG-sensitive sites (% DNA in tail) | 10.3 ± 1.2 |
| 8-OH-guanine (nM) | 25.3 ± 5.2 |
| 8-NO2-cGMP(nM) | 58.1 ± 13.9 |
| 8-OH-2′-deoxy-guanosine (nM) | 17.4 ± 2.3 |
| cGMP (nM) | 96.3 ± 4.9 |
| Markers of lipid peroxidation | |
| Oxylipins from Arachidonic Acid | |
| PGs | |
| D-Pathway | |
| 2,3-dinor-11β-PGF2 (µM) | 2.50 ± 0.24 |
| 11-β-PGF2α (µM) | 0.09 ± 0.01 |
| Tetranor PGDM (µM) | 0.40 ± 0.02 |
| PGDM (µM) | 0.45 ± 0.01 |
| Tetranor PGJM (µM) | 0 ± 0 |
| Tetranor PGDM lactone (µM) | 0 ± 0 |
| E-Pathway | |
| Tetranor PGAM (µM) | 6.38 ± 2.21 |
| Tetranor PGEM (µM) | 0.28 ± 0.02 |
| 20-OH-PGE2 (µM) | 0.27 ± 0.03 |
| PGE2 (µM) | 0 ± 0 |
| F-Pathway | |
| Tetranor PGFM (µM) | 0.21 ± 0.04 |
| PGF2α (µM) | 0.16 ± 0.03 |
| 20-OH-PGF2α (µM) | 3.47 ± 0.32 |
| 19(R)-OH-PGF2α (µM) | 0 ± 0 |
| I-Pathway | |
| 6-keto-PGF1α (µM) | 0 ± 0 |
| F2-IsoPs | |
| 15 series | |
| 2,3-dinor-15-F2t-IsoP (2,3-dinor-8-iso-PGF2α) (µM) | 0.90 ± 0.01 |
| 15-epi-15-F2t-IsoP (8-iso-15(R)-PGF2α) (µM) | 1.04 ± 0.05 |
| 15-F2t-IsoP (8-iso-PGF2α) (µM) | 0.09 ± 0.01 |
| 9-epi-15-F2t-IsoP (8-iso-PGF2β) (µM) | 0.02 ± 0.00 |
| 15-keto-15-F2t-IsoP (8-iso-15-keto-PGF2α) (µM) | 0.96 ± 0.00 |
| ent-15-epi-15-F2t-IsoP (Ent-8-iso-15S-PGF2α) (µM) | 0.61 ± 0.02 |
| 2,3-dinor-15-epi-15F2t (µM) | 1.51 ± 0.27 |
| 5 series | |
| 5-F2t (µM) | 5.89 ± 0.73 |
| 5-epi-5F2t (µM) | 1.32 ± 0.08 |
| E2-IsoPs | |
| 15 series | |
| 15-keto-15-E2t-IsoP (8-iso-15keto-PGE2) (µM) | 1.03 ± 0.11 |
| Oxylipins from Dihomo-γ-linolenic acid | |
| PGs | |
| PGE1 (µM) | 0.54 ± 0.03 |
| PGD2 (µM) | 2.02 ± 0.01 |
| PGF1α (µM) | 0.48 ± 0.04 |
| IsoPs | |
| 15-F1t-IsoP (8-iso-PGF1α) (µM) | 0.003 ± 0.000 |
| 15-E1t-IsoP (8-iso-PGE1) (µM) | 4.84 ± 0.68 |
| Variable | 1C | 3C | PC | p-Value # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value * | Pre | Post | p-Value * | Pre | Post | p-Value * | ||
| DNA strand breaks (% DNA in tail, PBS) | 17.2 ± 1.2 | 15.5 ± 1.0 | 0.331 | 19.8 ± 1.6 | 18.3 ± 0.9 | 0.017 | 20.3 ± 1.3 | 19.6 ± 0.8 | 0.005 | 0.349 |
| H2O2-induced DNA damage (% DNA in tail) | 12.4 ± 0.8 | 9.8 ± 0.8 | 0.155 | 12.6 ± 1.2 | 11.2 ± 1.3 | 0.410 | 11.9 ± 1.3 | 12.7 ± 1.4 | 0.678 | 0.938 |
| DNA strand breaks (% DNA in tail, EB) | 20.5 ± 1.6 | 17.0 ± 1.0 | 0.076 | 20.8 ± 1.0 | 17.7 ± 1.0 | 0.147 | 21.2 ± 1.9 | 17.7 ± 1.0 | 0.106 | 0.988 |
| FPG-sensitive sites (% DNA in tail) | 7.8 ± 1.3 | 5.8 ± 0.7 | 0.178 | 7.2 ± 0.9 | 6.9 ± 1.1 | 0.858 | 7.6 ± 1.1 | 6.8 ± 1.0 | 0.622 | 0.423 |
| Variable | 1C | 3C | PC | p-Value # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value * | Pre | Post | p-Value * | Pre | Post | p-Value * | ||
| 8-OH-guanine (nM) | 19.0 ± 5.5 | 8.7 ± 3.7 | 0.130 | 16.2 ± 4.2 | 9.2 ± 2.7 | 0.168 | 10.7 ± 2.6 | 12.7 ± 3.5 | 0.642 | 0.692 |
| 8-NO2-cGMP(nM) | 55.2 ± 16.9 | 22.4 ± 13.3 | 0.136 | 37.3 ± 14.2 | 56.4 ± 21.7 | 0.467 | 47.1 ± 21.9 | 48.2 ± 18.6 | 0.971 | 0.413 |
| 8-OH-2′-deoxy-guanosine (nM) | 12.3 ± 2.6 | 11.0 ± 2.6 | 0.715 | 11.1 ± 2.6 | 13.2 ± 2.6 | 0.559 | 14.4 ± 2.5 | 12.2 ± 2.6 | 0.545 | 0.812 |
| cGMP (nM) | 101.6 ± 8.0 | 131.7 ± 35.6 | 0.415 | 94.9 ± 6.6 | 114.2 ± 8.6 | 0.082 | 132.2 ± 35.5 | 107.0 ± 5.6 | 0.487 | 0.583 |
| Series | 1C | 3C | PC | p-Value # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value * | Pre | Post | p-Value * | Pre | Post | p-Value * | ||
| E2-IsoPs 15-series | 0.84 ± 0.09 | 0.77 ± 0.07 | 0.546 | 0.79 ± 0.07 | 0.78 ± 0.08 | 0.959 | 0.84 ± 0.03 | 0.67 ± 0.03 | 0.116 | 0.395 |
| F2-IsoPs 15 series | 4.48 ± 0.32 | 4.93 ± 0.38 | 0.374 | 4.56 ± 0.32 | 4.75 ± 0.22 | 0.613 | 4.63 ± 0.27 | 4.73 ± 0.27 | 0.796 | 0.865 |
| F2-IsoPs 5 series | 8.35 ± 0.66 | 10.64 ± 1.26 | 0.116 | 7.64 ± 0.78 | 10.37 ± 0.86 | 0.023 | 8.28 ± 0.79 | 9.68 ± 0.78 | 0.215 | 0.767 |
| PGs D-Pathway | 4.77 ± 0.22 | 4.59 ± 0.16 | 0.507 | 4.86 ± 0.21 | 4.54 ± 0.12 | 0.190 | 4.62 ± 0.17 | 4.61 ± 0.20 | 0.987 | 0.946 |
| PGs E-Pathway | 5.36 ± 2.04 | 5.13 ± 2.44 | 0.943 | 3.90 ± 1.54 | 7.53 ± 2.82 | 0.266 | 8.78 ± 2.85 | 7.01 ± 3.86 | 0.716 | 0.780 |
| PGs F-Pathway | 1.26 ± 0.34 | 0.67 ± 0.19 | 0.142 | 1.67 ± 0.37 | 0.76 ± 0.18 | 0.034 | 1.94 ± 0.43 | 0.52 ± 0.15 | 0.003 | 0.669 |
| PGs I-Pathway | nd | nd | - | nd | nd | - | nd | nd | - | - |
| IsoPs from DGLA | 3.37 ± 0.59 | 3.10 ± 0.46 | 0.715 | 3.12 ± 0.47 | 3.14 ± 0.53 | 0.978 | 3.53 ± 0.67 | 2.40 ± 0.20 | 0.116 | 0.395 |
| PGs from DGLA | 2.90 ± 0.15 | 3.08 ± 0.04 | 0.248 | 2.88 ± 0.15 | 3.09 ± 0.05 | 0.182 | 3.08 ± 0.04 | 3.08 ± 0.04 | 0.921 | 0.812 |
| Variable | 1C | 3C | PC | p-Value # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value * | Pre | Post | p-Value * | Pre | Post | p-Value * | ||
| Oxylipins from Arachidonic Acid | ||||||||||
| PGs | ||||||||||
| D-Pathway | ||||||||||
| 2,3-dinor-11β-PGF2 | 1.70 ± 0.23 | 1.59 ± 0.13 | 0.668 | 1.84 ± 0.22 | 1.57 ± 0.10 | 0.275 | 1.65 ± 0.15 | 1.65 ± 0.18 | 0.977 | 0.898 |
| 11-β-PGF2α | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.067 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.172 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.067 | 0.885 |
| Tetranor PGDM | 0.34 ± 0.02 | 0.35 ± 0.00 | 0.662 | 0.33 ± 0.02 | 0.34 ± 0.00 | 0.728 | 0.37 ± 0.02 | 0.34 ± 0.00 | 0.049 | 0.233 |
| PGDM | 0.40 ± 0.02 | 0.41 ± 0.01 | 0.718 | 0.40 ± 0.02 | 0.40 ± 0.01 | 0.843 | 0.42 ± 0.01 | 0.41 ± 0.01 | 0.756 | 0.692 |
| Tetranor PGJM | nd | nd | - | nd | nd | - | nd | nd | - | - |
| Tetranor PGDM lactone | nd | nd | - | nd | nd | - | nd | nd | - | - |
| E-Pathway | ||||||||||
| Tetranor PGAM | 4.72 ± 2.06 | 4.77 ± 2.44 | 0.988 | 2.35 ± 0.84 | 7.17 ± 2.82 | 0.110 | 9.46 ± 3.03 | 6.65 ± 3.86 | 0.570 | 0.779 |
| Tetranor PGEM | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.879 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.806 | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.857 | 0.704 |
| 20-OH-PGE2 | 0.14 ± 0.03 | 0.11 ±0.01 | 0.332 | 0.15 ± 0.02 | 0.12 ± 0.03 | 0.106 | 0.18 ± 0.02 | 0.11 ± 0.01 | 0.015 | 0.743 |
| PGE2 | nd | nd | - | nd | nd | - | nd | nd | - | - |
| F-Pathway | ||||||||||
| Tetranor PGFM | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.436 | 0.11 ± 0.03 | 0.07 ± 0.02 | 0.186 | 0.12 ± 0.03 | 0.05 ± 0.01 | 0.046 | 0.276 |
| PGF2α | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.693 | 0.12 ± 0.02 | 0.12 ± 0.03 | 0.920 | 0.12 ± 0.02 | 0.10 ± 0.01 | 0.226 | 0.530 |
| 20-OH-PGF2α | 1.35 ± 0.36 | 0.51 ± 0.18 | 0.045 | 1.32 ± 0.34 | 0.57 ± 0.17 | 0.057 | 1.75 ± 0.41 | 0.38 ± 0.14 | 0.003 | 0.744 |
| 19(R)-OH-PGF2α | nd | nd | - | nd | nd | - | nd | nd | - | - |
| I-Pathway | ||||||||||
| 6-keto-PGF1α | nd | nd | - | nd | nd | - | nd | nd | - | - |
| F2-IsoPs | ||||||||||
| 15 series | ||||||||||
| 2,3-dinor-15-F2t-IsoP (2,3-dinor-8-iso-PGF2α) | 0.83 ± 0.04 | 0.86 ± 0.01 | 0.412 | 0.84 ± 0.04 | 0.86 ± 0.01 | 0.572 | 0.86 ± 0.01 | 0.86 ± 0.01 | 0.863 | 0.990 |
| 15-epi-15-F2t-IsoP (8-iso-15(R)-PGF2α) | 1.25 ± 0.08 | 1.60 ± 0.13 | 0.028 | 1.25 ± 0.02 | 1.60 ± 0.08 | 0.020 | 1.29 ± 0.07 | 1.54 ± 0.08 | 0.028 | 0.855 |
| 15-F2t-IsoP (8-iso-PGF2α) | 0.12 ± 0.01 | 0.16 ± 0.02 | 0.089 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.072 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.023 | 0.833 |
| 9-epi-15-F2t-IsoP (8-iso-PGF2β) | 0.02 ± 0.00 | 0.02 ± 0.02 | 0.090 | 0.02 ± 0.00 | 0.03 ± 0.03 | 0.076 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.582 | 0.393 |
| 15-keto-15-F2t-IsoP (8-iso-15-keto-PGF2α) | 0.91 ± 0.05 | 1.04 ± 0.09 | 0.184 | 0.91 ± 0.05 | 0.95 ± 0.00 | 0.319 | 0.91 ± 0.05 | 0.95 ± 0.00 | 0.888 | 0.339 |
| ent-15-epi-15-F2t-IsoP (ent-8-iso-15S-PGF2α) | 0.57 ± 0.03 | 0.73 ± 0.12 | 0.205 | 0.57 ± 0.03 | 0.66 ± 0.03 | 0.052 | 0.63 ± 0.03 | 0.62 ± 0.02 | 0.639 | 0.388 |
| 2,3-dinor-15-epi-15-F2t | 0.69 ± 0.22 | 0.51 ± 0.15 | 0.492 | 0.83 ± 0.23 | 0.50 ± 0.11 | 0.201 | 0.58 ± 0.16 | 0.58 ± 0.19 | 0.981 | 0.907 |
| 5 series | ||||||||||
| 5-F2t-IsoP | 6.51 ± 0.54 | 8.95 ± 1.16 | 0.065 | 6.45 ± 0.72 | 8.73 ± 0.80 | 0.040 | 6.73 ± 0.67 | 8.08 ± 0.74 | 0.184 | 0.776 |
| 5-epi-5F2t-IsoP | 1.43 ± 0.08 | 1.69 ± 0.10 | 0.052 | 1.40 ± 0.11 | 1.64 ± 0.06 | 0.069 | 1.48 ± 0.04 | 1.60 ± 0.04 | 0.057 | 0.650 |
| E2-IsoPs | ||||||||||
| 15 series | ||||||||||
| 15-keto-15-E2t-IsoP (8-iso-15-keto-PGE2) | 0.81 ± 0.09 | 0.77 ± 0.07 | 0.714 | 0.69 ± 0.07 | 0.78 ± 0.08 | 0.380 | 0.90 ± 0.11 | 0.67 ± 0.03 | 0.045 | 0.395 |
| Oxylipins from Dihomo-γ-linolenic acid | ||||||||||
| PGs | ||||||||||
| PGE1 | 0.55 ± 0.03 | 0.61 ± 0.01 | 0.100 | 0.53 ± 0.04 | 0.60 ± 0.01 | 0.106 | 0.58 ± 0.01 | 0.59 ± 0.01 | 0.142 | 0.496 |
| PGD2 | 1.95 ± 0.10 | 2.08 ± 0.01 | 0.197 | 1.95 ± 0.10 | 2.08 ± 0.01 | 0.229 | 2.05 ± 0.01 | 2.06 ± 0.01 | 0.530 | 0.490 |
| PGF1α | 0.39 ± 0.03 | 0.39 ± 0.02 | 0.967 | 0.39 ± 0.03 | 0.43 ± 0.03 | 0.337 | 0.45 ± 0.03 | 0.42 ± 0.03 | 0.394 | 0.482 |
| IsoPs | ||||||||||
| 15-F1t-IsoP (8-iso-PGF1α) | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.590 | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.158 | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.491 | 0.382 |
| 15-E1t-IsoP (8-iso-PGE1) | 3.41 ± 0.59 | 3.10 ± 0.46 | 0.672 | 2.70 ± 0.38 | 3.14 ± 0.53 | 0.509 | 3.92 ± 0.7 | 2.40 ± 0.20 | 0.045 | 0.395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, D.; Domínguez-Perles, R.; Rosi, A.; Tassotti, M.; Angelino, D.; Medina, S.; Ricci, C.; Guy, A.; Oger, C.; Gigliotti, L.; et al. Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study. Nutrients 2021, 13, 2399. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072399
Martini D, Domínguez-Perles R, Rosi A, Tassotti M, Angelino D, Medina S, Ricci C, Guy A, Oger C, Gigliotti L, et al. Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study. Nutrients. 2021; 13(7):2399. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072399
Chicago/Turabian StyleMartini, Daniela, Raúl Domínguez-Perles, Alice Rosi, Michele Tassotti, Donato Angelino, Sonia Medina, Cristian Ricci, Alexandre Guy, Camille Oger, Letizia Gigliotti, and et al. 2021. "Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study" Nutrients 13, no. 7: 2399. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072399