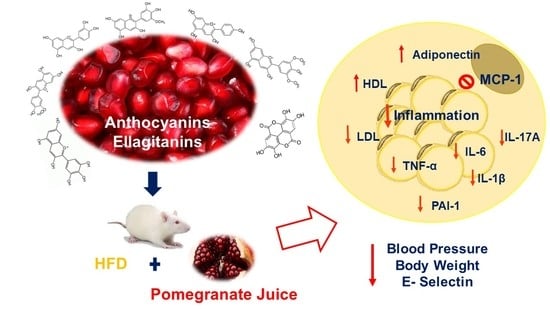
Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Obtaining the Pomegranate Juice
2.3. Quantification of Total Anthocyanins
2.4. Determination of Total Phenols
Phenol Compounds Identification
2.5. Animal Testing
2.6. Food and Energy Consumption and Body Weight
2.7. Adipose Tissue Extraction
2.8. Insulin Tolerance
2.9. Determining Systolic and Diastolic Blood Pressure
2.10. Lipid Profile
2.11. Determining Vascular Markers
2.12. Determining Inflammation Markers
2.13. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition, Phenolic Compounds
3.2. Effect of Pomegranate Juice on Physiological Parameters
| Parameters | Group SD | Group HD | Group JP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Food consumption (g/day) | 28.21 a | ± | 0.34 | 25.37 b | ± | 0.80 | 23.72 c | ± | 0.16 |
| Energy consumption (Kcal/day) | 87.45 a | ± | 1.07 | 113.5 b | ± | 3.87 | 106.78 c | ± | 0.70 |
| Gain in body weight (g) | 212.6 a | ± | 4.26 | 312.5 b | ± | 8.13 | 234.62 a | ± | 7.30 |
| Retroperitoneal adipose tissue (%) | 1.9 a | ± | 0.03 | 3.8 b | ± | 0.12 | 3.24 c | ± | 0.09 |
| Epididymal adipose tissue (%) | 1.48 a | ± | 0.04 | 2.59 b | ± | 0.18 | 2.67 b | ± | 0.08 |
| Glucose (mg/dL) | 112.3 a | ± | 0.97 | 128.2 b | ± | 3.49 | 115.5 a | ± | 2.95 |
| Systolic pressure (mmHg) | 117.7 a | ± | 1.26 | 141.9 b | ± | 1.68 | 112.61 a | ± | 0.16 |
| Diastolic pressure (mmHg) | 90.33 a | ± | 2.67 | 116.7 b | ± | 2.46 | 88.89 a | ± | 2.69 |
3.3. Lipid Profile
| Parameters | Group SD | Group HD | Group JP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lipidic ProFile # | Cholesterol (mg/dL) | 38.66 a | ± | 0.70 | 54.76 b | ± | 2.51 | 54.76 b,c | ± | 2.08 |
| cHDL (mg/dL) | 20.68 a | ± | 0.71 | 22.48 a | ± | 1.75 | 28.35 b | ± | 1.12 | |
| cLDL (mg/dL) | 10.13 a | ± | 0.90 | 15.60 b | ± | 1.91 | 9.54 a,c | ± | 0.95 | |
| Triglycerides (mg/dL) | 60.44 a | ± | 2.69 | 132.09 b | ± | 3.40 | 142.30 c | ± | 10.98 | |
| Vascular Markers * | E- selectin (ng/mL) | 8.63 a | ± | 0.15 | 11.03 b | ± | 0.44 | 6.27 c | ± | 0.04 |
| s-ICAM-1 (ng/mL) | 7.71 a | ± | 0.21 | 7.83 a | ± | 0.24 | 7.36 a | ± | 0.11 | |
| VWF (ng/mL) | 8.64 a | ± | 0.24 | 9.06 a | ± | 0.34 | 10.9 b | ± | 0.49 | |
| Adiponectin (ng/mL) | 70.39 a | ± | 2.36 | 58.97 b | ± | 1.48 | 69.37 a | ± | 1.17 | |
3.4. Vascular Parameters
3.5. Markers of Inflammation and Cytokines in Adipose Tissue


4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bulló, M.; Casas-Agustench, P.; Amigó-Correig, P.; Aranceta, J.; Salas-Salvadó, J. Inflammation, obesity and comorbidities: The role of diet. Public Health Nutr. 2007, 10, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Jarolimova, J.; Tagoni, J.; Stern, T.A. Obesity: Its epidemiology, comorbidities, and management. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.M.; Levings, M.K. Immune regulation in obesity-assosiated adipose inflammation. J. Immunol. 2013, 191, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanims linking obestity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Lima Vieira, R.A.; Nascimiento de Freitas, R.; Pinheiro, V. Adhesion molecules and chemokines: Relation to anthropometric, body composition, biochemical and dietary variables. Nutr. Hosp. 2014, 30, 223–236. [Google Scholar]
- Moreno Esteban, B.; Foz Sala, M. La Obesidad Como Factor de Riesgo Cardiovascular. Obesidad y Riesgo Cardiovascular; Estudio Dorica; Médica Panamericana: Madrid, Spain, 2004; pp. 49–60. [Google Scholar]
- Crespo, I.; García-Mediavilla, M.V.; Gutiérrez, B.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008, 100, 968–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Lovren, F.; Teoh, H.; Verma, S. Obesity and Atherosclerosis: Mechanistic Insights. Can. J. Cardiol. 2015, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Wang, Z. Inflammation, a link between obesity and cardiovascular disease. Mediat. Inflamm. 2010. [Google Scholar] [CrossRef] [Green Version]
- Arnold, N.; Lechner, K.; Shapiro, M.D.; Koening, W. Inflammation and Cardiovascular Disease. Eur. Cardiol. Rev. 2021, 16, e20. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, A.; Calhau, C. Pomegranate in human health: An overview. Bioact. Foods Promot. Health 2010, 551–563. [Google Scholar] [CrossRef]
- Khomich, L.M.; Perova, I.B.; Eller, K.I. Pomegranate juice nutritional profile. Vopr. Pitan. 2019, 88, 80–92. [Google Scholar]
- Hou, C.; Zhang, W.; Li, J.; Du, L.; Lv, O.; Zhao, S.; Li, J. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol. Nutr. Food Res. 2019, 63, 1–12. [Google Scholar] [CrossRef]
- De Nigris, F.; Williams-Ignarro, S.; Sica, V.; Lerman, L.O.; D’Armiento, F.P.; Byrns, R.E.; Napoli, C. Effects of a Pomegranate Fruit Extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc. Res. 2007, 73, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated pomegranate reverts high-density lipoprotein (hdl)-induced endothelial dysfunction and reduces postprandial triglyceridemia in women with acute coronary syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef] [Green Version]
- Giusti, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1–13. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef]
- Rabadan-Chávez, G.; Quevedo-Corona, L. Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. J. Funct. Foods 2016, 20, 54–67. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-garcía, Á.; Quevedo-corona, L.; Paniagua-castro, N.; Escalona-cardoso, G.; Reyes-maldonado, E.; Jaramillo-Flores, M.E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mphahlele, R.R.; Fawole, O.A.; Mokwena, L.M.; Opara, U.L. Effect of extraction method on chemical, volatile composition and antioxidant properties of pomegranate juice. S. Afr. J. Bot. 2016, 103, 135–144. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Labbé, M.; Ulloa, P.A.; Lopez, F.; Saenz, C.; Pena, A.; Salazar, F.N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (‘Wonderful’, ‘Chaca’ and ‘Codpa’) at different maturity stages. Chil. J. Agric. Res. 2016, 76, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Russo, M.; Cacciola, F.; Arena, K.; Mangraviti, D.; de Gara, L.; Dugo, P.; Mondello, L. Characterization of the polyphenolic fraction of pomegranate samples by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry detection. Nat. Prod. Res. 2019, 34, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sentandreu, E.; Cerdán-Calero, M.; Sendra, J.M. Phenolic profile characterization of pomegranate (Punica granatum) juice by high-performance liquid chromatography with diode array detection coupled to an electrospray ion trap mass analyzer. J. Food Compos. Anal. 2013, 30, 32–40. [Google Scholar] [CrossRef]
- Galván-Meléndez, M.F.; Lares-Bayona, E.F.; Quintanar-Escorza, M.A.; de la Ascención Carrera-Gracia, M.; Torres-Castorena, A. Concentraciones de leptina y su correlación con los componentes del síndrome metabólico y con el índice de masa corporal. Revista Biomédica 2014, 25, 23–30. [Google Scholar]
- Ahmed, M.M.; Samir, E.S.A.; El-Shehawi, A.M.; Alkafafy, M.E. Anti-obesity effects of Taif and Egyptian pomegranates: Molecular study. Biosci. Biotechnol. Biochem. 2015, 79, 598–609. [Google Scholar] [CrossRef]
- Simón, E.; Del Barrio, A.S. Leptina y obesidad. Anales del Sistema Sanitario de Navarra 2002, 25, 53–64. [Google Scholar] [PubMed] [Green Version]
- Miguelsanz, M.J.P.; Parra, W.C.; Moreiras, G.V.; Garaulet, M. Distribución regional de la grasa corporal: Uso de Técnicas de Imagen como Herramienta de Diagnóstico Nutricional. Nutr. Hosp. 2010, 25, 207–223. [Google Scholar]
- Ramos-Ibáñez, N. Tejido adipose intra-abdominal: Crecimiento, evaluación y su asociación con el Desarrollo de problemas metabólicos en niños y adolescents. Boletín Médico Hospital Infantil México 2009, 66, 492–501. [Google Scholar]
- Murphy, J.; Bacon, S.L.; Morais, J.A.; Tsoukas, M.A.; Santosa, S. Intra-Abdominal Adipose Tissue Quantification by Alternative Versus Reference Methods: A Systematic Review and Meta-Analysis. Obesity 2019, 27, 1115–1122. [Google Scholar] [CrossRef]
- Mertens, I.L.; Van Gaal, L.F. Overweight, obesity, and blood pressure: The effects of modest weight reduction. Obes. Res. 2000, 8, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Valido Díaz, A.; Romero Borges, R.; Bernal Llerena, T.; Fimia Duarte, R.; Iannacone, J. Biomodelo De Hipertensión Arterial En Ratas Wistar Administradas Con Solución Salina Al 10%. Biotempo 2018, 15, 75–82. [Google Scholar] [CrossRef]
- Flores Chávez, P.L.; Santos Martínez, L.E.; Martínez Memije, R.; Cortés Tenorio, S.; Sánchez Torres, G.; Vázquez Infante, O.I. Confiabilidad de la presion arterial sistémica determinada por un método no invasivo en ratas normotensas. Revista Instituto Nacional Enfermedades Respiratorias 2007, 20, 247–254. [Google Scholar]
- Barber Fox, M.O.; Barber Gutiérrez, E.; Fox Pascual, M. Hipertensión arterial experimental por medio del uso de un bloqueador competitive de angiotensina II. Revista Cubana Investigaciones Biomédicas 2006, 25, 1. [Google Scholar]
- Christerson, M. Anthocyanins and their effects on blood pressure. Fac. Nat. Res. Agric. Sci. Dev. Food Sci. 2016, 439, 1–23. [Google Scholar]
- Jordão, J.B.R.; Porto, H.K.P.; Lopes, F.M.; Batista, A.C.; Rocha, M.L. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J. Adipokines influence the inflammatory balance in autoimmunity. Cytokine 2015, 75, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Ferguson, L.R. Could pomegranate juice help in the control of inflammatory diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [Green Version]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. Int. Rev. J. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins Inhibit Nuclear Factor-κB Activation in Monocytes and Reduce Plasma Concentrations of Pro-Inflammatory Mediators in Healthy Adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Masana, L.; Ibarretxe, D.; Plana, N. Máxima reducción de colesterol unido a lipoproteínas de baja densidad alcanzable con combinaciones farmacológicas. Cuando 50 más 20 suma 60. Revista Española Cardiología 2016, 69, 342–343. [Google Scholar] [CrossRef]
- Jones, P.; Kafonek, S.; Laurora, I.; Hunninghake, D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (The Curves Study). Am. J. Cardiol. 1998, 81, 582–587. [Google Scholar] [CrossRef]
- Alfonso, J.E.F.; Ariza, I.D.S. Elevando el colesterol HDL: ¿Cuál es la mejor estrategia? Revista Associacao Medica Brasileira 2008, 54, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, O.; Sánchez-Madrid, F. Bases moleculares de las interacciones leucocito-endotelio durante la respuesta inflamatoria. Revista Española Cardiología 2009, 62, 552–562. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, E38–E81. [Google Scholar] [CrossRef] [Green Version]
- Engin, A. Adiponectin-Resistance in obesity. Obes. Lipotoxicity 2017, 960, 221–236. [Google Scholar]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Vincenzo, D.; Carbone, A.; et al. The role on von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwi, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Elissondo, N.; Rosso, L.G.; Maidana, P.; Brites, F. Bioquímica Clínica Actualización Adiponectina: Una adipocitoquina con múltiples funciones protectoras* Adiponectin: An adipocytokine with multiple protective functions. Acta Bioquímica Clínica Latinoamerica 2008, 24, 17–33. [Google Scholar]
- Domínguez Reyes, C. Adiponectina: El tejido adipose más allá de la reserve de energía. Revista Endocrinología Nutrición 2007, 15, 149–155. [Google Scholar]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef]
- Morales Clavijo, M.; Carvajal Garcés, C.F. Obesity and leptin resistance. Gac. Med. Bol. 2010, 33, 63–68. [Google Scholar]
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin applications in 2015: What have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Ploplis, V. Effects of Altered Plasminogen Activator Inhibitor-1 Expression on Cardiovascular Disease. Curr. Drug Targets 2011, 12, 1782–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthorn, W.P.; Sethi, J.K. TNF-α and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.; Reynisdottir, S.; Lönnqvist, F.; Stemme, V.; Hamsten, A.; Amer, P. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetología 1998, 41, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, M.; Salcedo, M.; Vázquez-Ortiz, G. Activación del Sistema Plasminógeno-Plasmina y el Papel de PAI-1 en Patologías Humanas. Revista Cancerologia. 2007, 2, 2. [Google Scholar]
- Bastarrachea, R.A.; López-Alvarenga, J.C.; Bolado-garcía, V.E.; Téllez-mendoza, J.; Comuzzie, A.G. Obesidad y resistencia a la insulina. Gaceta Médica México 2007, 143, 505–512. [Google Scholar]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Mohanty, P.; Aljada, A.; Ghanim, H.; Hofmeyer, D.; Tripathy, D.; Syed, T.; Al-Haddad, W.; Dhindsa, S.; Dandona, P. Evidence for a potent antiinflammatory effect of rosiglitazone. J. Clin. Endocrinol. Metab. 2004, 89, 2728–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higa, J.K.; Liu, W.; Berry, M.J.; Panee, J. Supplement of bamboo extract lowers serum monocyte chemoattractant protein-1 concentration in mice fed a diet containing a high level of saturated fat. Br. J. Nutr. 2011, 106, 1810–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.H.; Tsuyoshi, G.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.R.; Ceperuelo-Mallafré, V.; Maymó-Masip, E.; Mateo-Sanz, J.M.; Arola, L.; Guitiérrez, C.; Fernández-Real, J.M.; Ardèvol, A.; Simón, I.; Vendrell, J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine 2009, 47, 137–142. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, J.M.; Joung, K.H.; Lee, J.H.; You, B.R.; Choi, M.J.; Ryu, M.J.; Ko, Y.B.; Lee, A.M.; Lee, J.; et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS ONE 2016, 11, e0154003. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Hacohen, N.; Golub, T.R.; Parijs, L.; Van Lodish, H.F. Tumor Necrosis Factor-α Suppresses Adipocyte-Specific Genes and Activates Expression of Preadipocyte Genes in 3T3-L1 Adipocytes. Cell Rep. 2002, 1, 1319–1336. [Google Scholar]
- Weisberg, S.P.; Leibel, R.L.; Anthony, W.F., Jr.; Weisberg, S.P.; Mccann, D.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. Find the latest version: Obesity is associated with. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, S.; Lati, Y.; Aviram, M.; Fuhrman, B. Pomegranate juice polyphenols induce a phenotypic switch in macrophage polarization favoring a M2 anti-inflammatory state. BioFactors 2015, 41, 44–51. [Google Scholar] [CrossRef]
- Bradley, R.L.; Fisher, F.M.; Maratos-Flier, E. Dietary fatty acids differentially regulate production of TNF-α and IL-10 by murine 3T3-L1 adipocytes. Obesity 2008, 16, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Fain, J.N. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: A review. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lona, J.M.F.; Martínez, M.S.; Alarcón, G.V.; Rodas, B.A.; Bello, J.R. El factor de necrosis tumoral α (TNF-α) en las enfermedades cardovasculares: Biología Molecular y Genética. Gaceta Médica México 2014, 150, 334–344. [Google Scholar]
- Ramírez Alvarado, M.; Sánchez Roitz, C. Tumor necrosis factor-α, insulin resistance, the lipoprotein metabolism and obesity in humans. Nutr. Hosp. 2012, 27, 1751–1757. [Google Scholar]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE 2012, 7, e29837. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary Cyanidin 3-O-β-D-Glucoside-Rich Purple Corn Color Prevents Obesity and Ameliorates Hyperglycemia in Mice. J. Nutr. 2018, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michicotl-Meneses, M.M.; Thompson-Bonilla, M.d.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats. Nutrients 2021, 13, 2577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082577
Michicotl-Meneses MM, Thompson-Bonilla MdR, Reyes-López CA, García-Pérez BE, López-Tenorio II, Ordaz-Pichardo C, Jaramillo-Flores ME. Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats. Nutrients. 2021; 13(8):2577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082577
Chicago/Turabian StyleMichicotl-Meneses, Maria Monica, María del Rocío Thompson-Bonilla, César A. Reyes-López, Blanca Estela García-Pérez, Itzel I. López-Tenorio, Cynthia Ordaz-Pichardo, and María Eugenia Jaramillo-Flores. 2021. "Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats" Nutrients 13, no. 8: 2577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082577