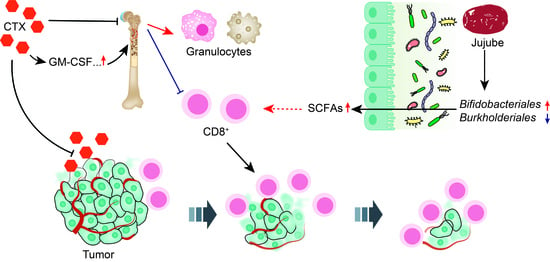
Jujube Powder Enhances Cyclophosphamide Efficiency against Murine Colon Cancer by Enriching CD8+ T Cells While Inhibiting Eosinophilia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Mice and Treatment
2.3. DNA Extraction and Bacterial Identification in Stool Samples
2.4. Bioinformatics Analysis
2.5. Complete Blood Counts
2.6. Flow Cytometry of Blood, Bone, Spleen, and Tumor Populations
2.7. Staining
2.8. Determination of Cytokines in Serum
2.9. Quantification of SCFAs
2.10. Statistical Analysis
3. Results
3.1. Jujube Facilitates Tumor-Infiltrating CD8+ T Cells and Enhances CTX Efficiency
3.2. Restoration of Gut Microbiota with Jujube
3.2.1. Jujube Increased Gut Microbiota Diversity
3.2.2. Jujube Powder Altered the Composition of Gut Microbiota
3.2.3. Function Differences in the Gut Microbiota
3.3. Jujube Powder Increased the Production of SCFAs
3.4. Jujube Powder Enriched CD8+ T Cells but Reduced Eosinophilia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-induced neutropenia—Risks, consequences, and new directions for its management. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef]
- Taylor, S.J.; Duyvestyn, J.M.; Dagger, S.A.; Dishington, E.J.; Rinaldi, C.A.; Dovey, O.M.; Vassiliou, G.S.; Grove, C.S.; Langdon, W.Y. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci. Transl. Med. 2017, 9, eaam8060. [Google Scholar] [CrossRef] [Green Version]
- Toledo, M.; Penna, F.; Oliva, F.; Luque, M.; Betancourt, A.; Marmonti, E.; Lopez-Soriano, F.J.; Argiles, J.M.; Busquets, S. A multifactorial anti-cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J. Cachexia Sarcopenia Muscle 2016, 7, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; des Varannes, S.B.; Le Vacon, F.; de La Cochetiere, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Vanhoefer, U.; Harstrick, A.; Achterrath, W.; Cao, S.S.; Seeber, S.; Rustum, Y.M. Irinotecan in the treatment of colorectal cancer: Clinical overview. J. Clin. Oncol. 2001, 19, 1501–1518. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.J.; Hamilton, J.; Keefe, D.M.K. Gastrointestinal Microflora and Mucins May Play a Critical Role in the Development of 5-Fluorouracil-Induced Gastrointestinal Mucositis. Exp. Biol. Med. 2009, 234, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S.; et al. Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Voorde, J.; Sabuncuoglu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing Enzymes in Mycoplasma-infected Tumor Cell Cultures Compromise the Cytostatic Activity of the Anticancer Drug Gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, J.M.; Stringer, A.M.; Gibson, R.J.; Yeoh, A.S.J.; Hannam, S.; Keefe, D.M.K. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol. Ther. 2007, 6, 1449–1454. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Redman, M.G.; Ward, E.J.; Phillips, R.S. The efficacy and safety of probiotics in people with cancer: A systematic review. Ann. Oncol. 2014, 25, 1919–1929. [Google Scholar] [CrossRef]
- Schoener, C.A.; Carillo-Conde, B.; Hutson, H.N.; Peppas, N.A. An inulin and doxorubicin conjugate for improving cancer therapy. J. Drug Deliv. Sci. Technol. 2013, 23, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ward, K.K.; Shah, N.R.; Saenz, C.C.; McHale, M.T.; Plaxe, S.C. Excess risk of Clostridium difficile infection in ovarian cancer is related to exposure to broad-spectrum antibiotics. Supportive Care Cancer 2013, 21, 3103–3107. [Google Scholar] [CrossRef] [PubMed]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437. [Google Scholar] [CrossRef]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Supportive Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Luo, X.; Zhang, B.; Song, W.-X.; Mehendale, S.; Xie, J.-T.; Aung, H.H.; He, T.-C.; Yuan, C.-S. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother. Pharmacol. 2007, 60, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Z.; Maiwulanjiang, M.; Zhang, W.L.; Zhan, J.Y.X.; Lam, C.T.W.; Zhu, K.Y.; Yao, P.; Choi, R.C.Y.; Lau, D.T.W.; et al. Chemical and Biological Assessment of Ziziphus jujuba Fruits from China: Different Geographical Sources and Developmental Stages. J. Agric. Food Chem. 2013, 61, 7315–7324. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Yen, G.-C.; Sheu, F.; Chau, C.-F. Effects of water-soluble carbohydrate concentrate from Chinese jujube on different intestinal and fecal indices. J. Agric. Food Chem. 2008, 56, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, X.; Han, A.; Chen, P.; Bai, H. In vitro immunological and anti-complementary activities of two water-soluble lignins from Zizyphus jujube cv. Jinchangzao. Int. J. Biol. Macromol. 2017, 105, 204–212. [Google Scholar] [CrossRef]
- Wang, L.; Jing, N.; Liu, X.; Jiang, G.; Liu, Z. Nurturing and modulating gut microbiota with jujube powder to enhance anti-PD-L1 efficiency against murine colon cancer. J. Funct. Foods 2020, 64, 103647. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating Gut Microbiome with Bilberry Anthocyanin Combo to Enhance Anti-PD-L1 Efficiency against Murine Colon Cancer. Microorganisms 2020, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Nurrochmad, A.; Ikawati, M.; Sari, I.P.; Murwanti, R.; Nugroho, A.E. Immunomodulatory Effects of Ethanolic Extract of Thyphonium flagelliforme (Lodd) Blume in Rats Induced by Cyclophosphamide. J. Evid. Based Integr. Med. 2015, 20, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Escudero-Vilaplana, V.; Osorio-Prendes, S.; Collado-Borrell, R.; Gonzalez-Arias, E.; Sanjurjo-Saez, M. Eosinophilia secondary to lenalidomide therapy. J. Clin. Pharm. Ther. 2018, 43, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8+ T-cell responses and immune memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.E.; Spasova, D.S.; Frimpong-Boateng, K.; Kim, H.-O.; Lee, M.; Kim, K.S.; Surh, C.D. Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 2017, 47, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Serino, M. SCFAs—The thin microbial metabolic line between good and bad. Nat. Rev. Endocrinol. 2019, 15, 318–319. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021, 33, 988–1000. [Google Scholar] [CrossRef]






| Cells | Markers |
|---|---|
| Total T cells | CD45+ |
| CD4+ T cells | CD45+CD4+CD8− |
| CD8+ T cells | CD45+CD8+CD4− |
| B cells | CD19+ |
| Myeloid | CD11b+ |
| Eosinophils | CD11b+Ly6G−F4/80+SSCh |
| Monocytes | CD11b+Ly6C+ Ly6G−SSCl |
| Macrophages | CD11b+Ly6C−Ly6G−SSCl |
| Granulocytes | CD11b+Ly6G+SSCm |
| Markers | Conjugate | Manufacturer |
|---|---|---|
| CD45 | APCCy7 | BioLegend |
| CD4 | PECy7 | BioLegend |
| CD8a | BrilliantViolet605 | BioLegend |
| CD19 | AlexaFluor700 | BioLegend |
| Ly6C | PE | BioLegend |
| Ly6G | FITC | BioLegend |
| F4/80 | BrilliantViolet421 | BioLegend |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, H.; Jing, N.; Wang, L.; Jiang, G.; Liu, Z. Jujube Powder Enhances Cyclophosphamide Efficiency against Murine Colon Cancer by Enriching CD8+ T Cells While Inhibiting Eosinophilia. Nutrients 2021, 13, 2700. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082700
Zhuang H, Jing N, Wang L, Jiang G, Liu Z. Jujube Powder Enhances Cyclophosphamide Efficiency against Murine Colon Cancer by Enriching CD8+ T Cells While Inhibiting Eosinophilia. Nutrients. 2021; 13(8):2700. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082700
Chicago/Turabian StyleZhuang, Huiren, Nan Jing, Luoyang Wang, Guoqiang Jiang, and Zheng Liu. 2021. "Jujube Powder Enhances Cyclophosphamide Efficiency against Murine Colon Cancer by Enriching CD8+ T Cells While Inhibiting Eosinophilia" Nutrients 13, no. 8: 2700. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082700