Serum 25-Hydroxyvitamin D Concentrations and Atopic Dermatitis in Early Childhood: Findings from the Japan Environment and Children’s Study
Abstract
:1. Introduction
2. Methods
2.1. Population and Variables
2.2. Statistical Analysis
3. Results
3.1. Participant Characteristics
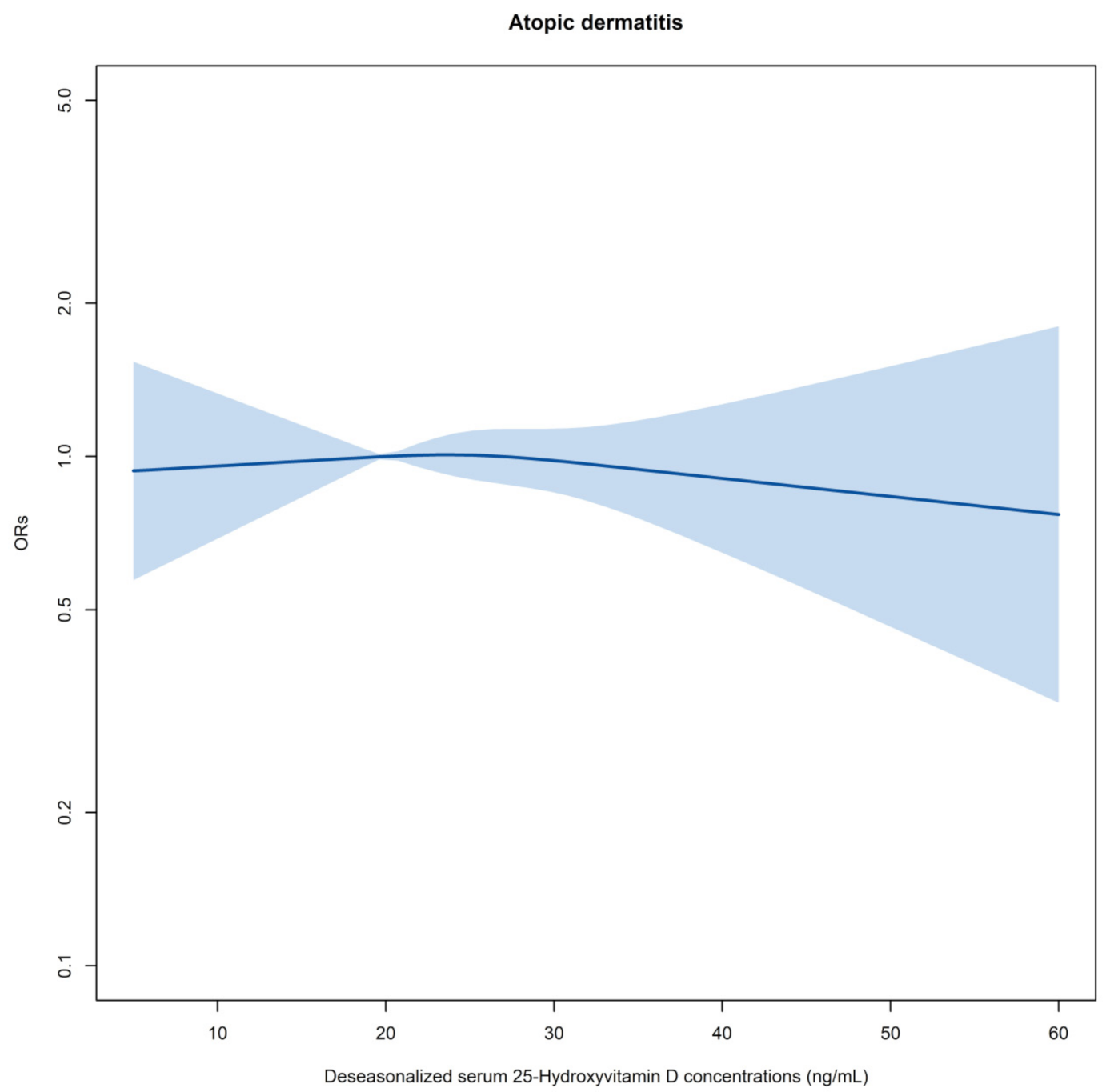
3.2. VitD and Childhood Atopic Dermatitis
3.3. Sensitivity Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gazibara, T.; Elbert, N.J.; den Dekker, H.T.; de Jongste, J.C.; Reiss, I.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Tiemeier, H.; Jaddoe, V.W.; et al. Associations of maternal and fetal 25-hydroxyvitamin D levels with childhood eczema: The Generation R Study. Pediatr. Allergy Immunol. 2016, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manage. Care 2017, 23, S115–S123. [Google Scholar]
- Yamamoto-Hanada, K.; Yang, L.; Saito-Abe, M.; Sato, M.; Inuzuka, Y.; Toyokuni, K.; Nishimura, K.; Irahara, M.; Ishikawa, F.; Miyaji, Y.; et al. Four phenotypes of atopic dermatitis in Japanese children: A general population birth cohort study. Allergol. Int. 2019, 68, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Pak, K.; Saito-Abe, M.; Yang, L.; Sato, M.; Irahara, M.; Mezawa, H.; Sasaki, H.; Nishizato, M.; Ishitsuka, K.; et al. Allergy and immunology in young children of Japan: The JECS cohort. World Allergy Organ. J. 2020, 13, 100479. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Sanmartin, R.; Pardos, C.; Doste, D.; Aguilera, J.; Alijarde, R.; Agon-Banzo, P.J.; Garcia-Malinis, A.J.; Puzo, J.; Hernandez-Martin, A.; Gilaberte, Y. The association between atopic dermatitis and serum 25-hydroxyvitamin D in children: Influence of sun exposure, diet, and atopy features—A cross-sectional study. Pediatr. Dermatol. 2020, 37, 294–300. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, H.F.; Schnoes, H.K. Vitamin D: Recent advances. Annu. Rev. Biochem. 1983, 52, 411–439. [Google Scholar] [CrossRef]
- Chung, M.; Balk, E.M.; Brendel, M.; Ip, S.; Lau, J.; Lee, J.; Lichtenstein, A.; Patel, K.; Raman, G.; Tatsioni, A.; et al. Vitamin D and calcium: A systematic review of health outcomes. Evid. Rep. Technol. Assess. 2009, 183, 1–420. [Google Scholar]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J. Am. Acad. Dermatol. 2013, 69, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Wills, A.K.; Shaheen, S.O.; Granell, R.; Henderson, A.J.; Fraser, W.D.; Lawlor, D.A. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin. Exp. Allergy 2013, 43, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D metabolism and function in the skin. Mol. Cell. Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Yue, H.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Chieosilapatham, P.; Kiatsurayanon, C.; Song, P.; Okumura, K.; Ogawa, H.; et al. Exogenous factors in the pathogenesis of atopic dermatitis: Irritants and cutaneous infections. Clin. Exp. Allergy 2021, 51, 382–392. [Google Scholar] [CrossRef]
- Palmer, D.J. Vitamin D and the Development of Atopic Eczema. J. Clin. Med. 2015, 4, 1036–1050. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A., Jr.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef]
- Oren, E.; Banerji, A.; Camargo, C.A., Jr. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J. Allergy Clin. Immunol. 2008, 121, 533–534. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Muehleisen, B.; Gallo, R.L. Vitamin D in allergic disease: Shedding light on a complex problem. J. Allergy Clin. Immunol. 2013, 131, 324–329. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, Y.; Saijo, Y.; Yoshioka, E.; Nakagi, Y.; Yoshida, T.; Miyamoto, T.; Sengoku, K.; Ito, Y.; Miyashita, C.; Araki, A.; et al. The Association between Prenatal Yoga and the Administration of Ritodrine Hydrochloride during Pregnancy: An Adjunct Study of the Japan Environment and Children’s Study. PLoS ONE 2016, 11, e0158155. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yamamoto-Hanada, K.; Pak, K.; Ayabe, T.; Mezawa, H.; Ishitsuka, K.; Konishi, M.; Yang, L.; Matsumoto, K.; Saito, H.; et al. Having small-for-gestational-age infants was associated with maternal allergic features in the JECS birth cohort. Allergy 2018, 73, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Ishitsuka, K.; Pak, K.; Saito, M.; Ayabe, T.; Mezawa, H.; Konishi, M.; Yang, L.; Matsumoto, K.; Saito, H.; et al. Allergy and mental health among pregnant women in the Japan Environment and Children’s Study. J. Allergy Clin. Immunol. Pract. 2018, 6, 1421–1424.e2. [Google Scholar] [CrossRef]
- Komori, K.; Komori, M.; Eitoku, M.; Muchanga, S.M.J.; Ninomiya, H.; Kobayashi, T.; Suganuma, N.; Japan Environment and Children’s Study (JECS) Group. Verbal abuse during pregnancy increases frequency of newborn hearing screening referral: The Japan Environment and Children’s Study. Child Abus. Negl. 2019, 90, 193–201. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Irahara, M.; Nishizato, M.; Sasaki, H.; Konishi, M.; Ishitsuka, K.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Association of Hemoglobin and Hematocrit Levels during Pregnancy and Maternal Dietary Iron Intake with Allergic Diseases in Children: The Japan Environment and Children’s Study (JECS). Nutrients 2021, 13, 810. [Google Scholar] [CrossRef]
- Sekiyama, M.; Yamazaki, S.; Michikawa, T.; Nakayama, S.F.; Nitta, H.; Taniguchi, Y.; Suda, E.; Isobe, T.; Kobayashi, Y.; Iwai-Shimada, M.; et al. Study design and participants’ profile in the Sub-Cohort Study in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2020, JE20200448. [Google Scholar] [CrossRef]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Inadera, H.; The Japan Environment and Children’s Study (JECS) Group. House Dust Avoidance during Pregnancy and Subsequent Infant Development: The Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 2021, 18, 4277. [Google Scholar] [CrossRef]
- Minatoya, M.; Araki, A.; Miyashita, C.; Itoh, S.; Kobayashi, S.; Yamazaki, K.; Bamai, Y.A.; Saijyo, Y.; Ito, Y.; Kishi, R.; et al. Cat and Dog Ownership in Early Life and Infant Development: A Prospective Birth Cohort Study of Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 2019, 17, 205. [Google Scholar] [CrossRef] [Green Version]
- Van der Mei, I.A.; Ponsonby, A.L.; Dwyer, T.; Blizzard, L.; Taylor, B.V.; Kilpatrick, T.; Butzkueven, H.; McMichael, A.J. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J. Neurol. 2007, 254, 581–590. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Weiland, S.K.; Bjorksten, B.; Brunekreef, B.; Cookson, W.O.; von Mutius, E.; Strachan, D.P.; International Study of Asthma and Allergies in Childhood Phase II Study Group. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and methods. Eur. Respir. J. 2004, 24, 406–412. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; Beasley, R.; Clayton, T.O.; Stewart, A.W.; ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int. J. Tuberc. Lung Dis. 2005, 9, 10–16. [Google Scholar]
- Buuren, S.V.; Groothuis, O.K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/. (accessed on 8 August 2021).
- Boyle, V.T.; Thorstensen, E.B.; Thompson, J.M.D.; McCowan, L.M.E.; Mitchell, E.A.; Godfrey, K.M.; Poston, L.; Wall, C.R.; Murphy, R.; Cutfield, W.; et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: An observational study. Int. J. Obes. 2017, 41, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, A.; Hourihane, J.O.; Malvisi, L.; Irvine, A.D.; Kenny, L.C.; Murray, D.M.; Kiely, M.E. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE Birth Cohort Study. Allergy 2018, 73, 2182–2191. [Google Scholar] [CrossRef]
- Berents, T.L.; Carlsen, K.C.L.; Mowinckel, P.; Sandvik, L.; Skjerven, H.O.; Rolfsjord, L.B.; Kvenshagen, B.; Hunderi, J.O.; Bradley, M.; Lieden, A.; et al. Vitamin D levels and atopic eczema in infancy and early childhood in Norway: A cohort study. Br. J. Dermatol. 2016, 175, 95–101. [Google Scholar] [CrossRef]
- Cairncross, C.; Grant, C.; Stonehouse, W.; Conlon, C.; McDonald, B.; Houghton, L.; Eyles, D.; Camargo, C.A.; Coad, J.; von Hurst, P. The Relationship between Vitamin D Status and Allergic Diseases in New Zealand Preschool Children. Nutrients 2016, 8, 326. [Google Scholar] [CrossRef]
- Reinehr, T.; Langrock, C.; Hamelmann, E.; Lucke, T.; Koerner-Rettberg, C.; Holtmann, M.; Legenbauer, T.; Gest, S.; Frank, M.; Schmidt, B.; et al. 25-Hydroxvitamin D concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attention-deficient/hyperactivity disorder than in healthy children. Nutr. Res. 2018, 52, 39–47. [Google Scholar] [CrossRef]
- Heimbeck, I.; Wjst, M.; Apfelbacher, C.J. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy 2013, 68, 906–910. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C.; Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.P.; D’Vaz, N.; Meldrum, S.; Palmer, D.J.; Zhang, G.; Prescott, S.L. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin. Exp. Allergy 2015, 45, 220–231. [Google Scholar] [CrossRef]
- Koseki, R.; Morii, W.; Noguchi, E.; Ishikawa, M.; Yang, L.; Yamamoto-Hanada, K.; Narita, M.; Saito, H.; Ohya, Y. Effect of filaggrin loss-of-function mutations on atopic dermatitis in young age: A longitudinal birth cohort study. J. Hum. Genet. 2019, 64, 911–917. [Google Scholar] [CrossRef]
- Hattori, H.; Kitamura, A.; Takahashi, F.; Kobayashi, N.; Sato, A.; Miyauchi, N.; Nishigori, H.; Mizuno, S.; Sakurai, K.; Ishikuro, M.; et al. The risk of secondary sex ratio imbalance and increased monozygotic twinning after blastocyst transfer: Data from the Japan Environment and Children’s Study. Reprod. Biol. Endocrinol. 2019, 17, 27. [Google Scholar] [CrossRef]
- Saliba, W.; Barnett, O.; Stein, N.; Kershenbaum, A.; Rennert, G. The longitudinal variability of serum 25(OH)D levels. Eur. J. Intern. Med. 2012, 23, e106–e111. [Google Scholar] [CrossRef]

| 25(OH)D (ng/mL) | Odds Ratios (95% CI) $ | Odds Ratios (95% CI) & |
|---|---|---|
| ALL | ||
| <20 vs. ≥30 | 1.06 (0.83–1.35) | 0.97 (0.74–1.27) |
| ≥20 and <30 vs. ≥30 | 0.96 (0.77–1.19) | 0.92 (0.73–1.14) |
| Sub dataset # | ||
| <20 vs. ≥30 | 1.25 (0.79–1.98) | 1.23 (0.75–2.03) |
| ≥20 and <30 vs. ≥30 | 1.22 (0.82–1.83) | 1.21 (0.80–1.83) |
| Deseasonalized 25(OH)D | ORs (95% CI) $ | ORs (95% CI) & |
|---|---|---|
| All | ||
| Deseasonalized 25(OH)D category 1 vs. 3 | 0.91 (0.69–1.20) | 0.91 (0.69–1.21) |
| Deseasonalized 25(OH)D category 2 vs. 3 | 0.92 (0.70–1.21) | 0.95 (0.72–1.26) |
| Deseasonalized 25(OH)D category 4 vs. 3 | 0.92 (0.70–1.21) | 0.89 (0.67–1.17) |
| Deseasonalized 25(OH)D category 5 vs. 3 | 0.93 (0.71–1.22) | 0.94 (0.71–1.23) |
| Sub dataset # | ||
| Deseasonalized 25(OH)D category 1 vs. 3 | 0.89 (0.57–1.41) | 0.90 (0.57–1.42) |
| Deseasonalized 25(OH)D category 2 vs. 3 | 0.70 (0.43–1.14) | 0.72 (0.44–1.17) |
| Deseasonalized 25(OH)D category 4 vs. 3 | 0.80 (0.51–1.28) | 0.79 (0.50–1.27) |
| Deseasonalized 25(OH)D category 5 vs. 3 | 0.64 (0.39–1.05) | 0.65 (0.40–1.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Sato, M.; Saito-Abe, M.; Nishizato, M.; Mezawa, H.; Yamamoto-Hanada, K.; Ohya, Y.; The Japan Environment and Children’s Study (JECS) Group. Serum 25-Hydroxyvitamin D Concentrations and Atopic Dermatitis in Early Childhood: Findings from the Japan Environment and Children’s Study. Nutrients 2021, 13, 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082761
Yang L, Sato M, Saito-Abe M, Nishizato M, Mezawa H, Yamamoto-Hanada K, Ohya Y, The Japan Environment and Children’s Study (JECS) Group. Serum 25-Hydroxyvitamin D Concentrations and Atopic Dermatitis in Early Childhood: Findings from the Japan Environment and Children’s Study. Nutrients. 2021; 13(8):2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082761
Chicago/Turabian StyleYang, Limin, Miori Sato, Mayako Saito-Abe, Minaho Nishizato, Hidetoshi Mezawa, Kiwako Yamamoto-Hanada, Yukihiro Ohya, and The Japan Environment and Children’s Study (JECS) Group. 2021. "Serum 25-Hydroxyvitamin D Concentrations and Atopic Dermatitis in Early Childhood: Findings from the Japan Environment and Children’s Study" Nutrients 13, no. 8: 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082761






