Edible Plant Sprouts: Health Benefits, Trends, and Opportunities for Novel Exploration
Abstract
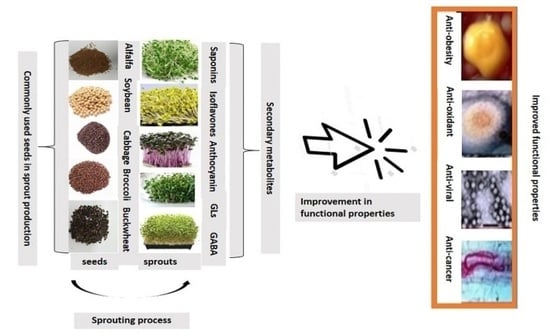
:1. Introduction
2. Germination Process of Seeds
3. Phytochemicals in Selected Edible Plant Sprouts
4. Health Benefits of Sprouts
4.1. Antioxidant Activity
4.2. Cytotoxic Activity
4.3. Antidiabetic Activity
4.4. Hypocholesterolemic and Anti-Obesity Activity
4.5. Antiviral Activity
4.6. Antiatherosclerosis Activity
5. Recent Novel Approach for Enhancing Biological Activities of Sprouts
5.1. Application of Slightly Acidified Electrolyte Water as an Elicitor in Sprouts
5.2. Sucrose Treatment
5.3. Irradiation in Sprout Production
5.4. Computer-Based Prediction Approaches
5.5. Seed Priming
5.6. Sprout Biofortification
5.7. Hurdle Approach in Enhancing Functional Properties of Sprouts
6. Microbial Safety of Sprouts
6.1. Intervention Strategies for the Microbial Safety of Sprouts
6.2. Plasma-Based Treatments
6.3. Electrolyte Water (EW)
6.4. Ultrasound Treatment
6.5. Photosensitization
6.6. Use of Natural Oil (Essential Oils)
6.7. Proposed Guides to Reduce the Microbial Hazard in Sprouts
7. New Horizons in Sprout Studies
7.1. Could Metabolic Engineering or Biotransformation Be a Solution to the Diversity of Sprout Metabolites?
7.2. Green Synthesis of Nanoparticles Using Plant Sprouts
7.3. The Rise of Sprouting and Gut Health
7.4. Emerging Uses of Plant Sprouts in Processed Products
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end-products |
| EOs | Essential oils |
| EW | Electrolyte water |
| GLs | Glucosinolates |
| GABA | Gamma-aminobutyric acid |
| LED | Light-emitting diode |
| ME | Metabolic engineering |
| NBC | Natural bioactive compounds |
| PAL | Phenylalanine ammonia-lyase |
| ROS | Reactive oxygen species |
| SAEW | Slightly acidified electrolyte water |
| SFN | Sulforaphane |
References
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Waginger, H. Production and use of sprouted grains [barley, oats]. In Proceedings of the 6th International Congress on Soilless Culture, Lunteren, The Netherlands, 29 April–5 May 1984. [Google Scholar]
- Yilmaz, H.Ö.; Ayhan, N.Y.; Meriç, Ç.S. Buckwheat: A Useful Food and Its Effects on Human Health. Curr. Nutr. Food Sci. 2020, 16, 29–34. [Google Scholar] [CrossRef]
- Shah, M.A.; Sarker, M.M.R.; Gousuddin, M. Antidiabetic potential of Brassica Oleracea Var. Italica in type 2 diabetic sprague dawley (sd) rats. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 462–469. [Google Scholar]
- Shi, Y.H.; Wang, J.; Guo, R.; Wang, C.Z.; Yan, X.B.; Xu, B.; Zhang, D.Q. Effects of alfalfa saponin extract on growth per-formance and some antioxidant indices of weaned piglets. Livest. Sci. 2014, 167, 257–262. [Google Scholar] [CrossRef]
- Bastida, J.A.G.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Rifna, E.; Ramanan, K.R.; Mahendran, R. Emerging technology applications for improving seed germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Ertop, M.H.; Bektaş, M. Enhancement of Bioavailable Micronutrients and Reduction of Antinutrients in Foods with some Processes. Food Health 2018, 4, 159–165. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010, 121, 387–392. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Sauer, D.B.; Burroughs, R. Disinfection of seed surfaces with sodium hypochlorite. Phytopathology 1986, 76, 745–749. [Google Scholar] [CrossRef]
- Idowu, A.T.; Olatunde, O.O.; Adekoya, A.E.; Idowu, S. Germination: An alternative source to promote phytonutrients in edible seeds. Food Qual. Saf. 2020, 4, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, W.M. Muskmelon seed germination and seedling development in response to seed priming. Sci. Agric. 2003, 60, 71–75. [Google Scholar] [CrossRef]
- Dove, N. The Effect of Increasing Temperature on Germination of Native Plant Species in the North Woods Region; University of Vermont: Burlington, VT, USA, 2010. [Google Scholar]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative Study of the Bioactive Properties and Elemental Composition of Red Clover (Trifolium pratense) and Alfalfa (Medicago sativa) Sprouts during Germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- El-Deeb, M.M.K.; El-Sheredy, H.G.; Mohammed, A.F. The role of serum trace elements and oxidative stress in egyptian breast cancer patients. Adv. Breast Cancer Res. 2016, 5, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Amraie, E.; Farsani, M.K.; Sadeghi, L.; Khan, T.N.; Babadi, V.Y.; Adavi, Z. The effects of aqueous extract of alfalfa on blood glucose and lipids in alloxan-induced diabetic rats. Interv. Med. Appl. Sci. 2015, 7, 124–128. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Hassan, A.H.A.; Al Jaouni, S.K.; Alkhalifah, D.H.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H.; Khamis, G. Influence of elevated CO2 on nutritive value and health-promoting prospective of three genotypes of Alfalfa sprouts (Medicago Sativa). Food Chem. 2020, 340, 128147. [Google Scholar] [CrossRef]
- Hamilton, M.J.; VanderStoep, J. Germination and Nutrient Composition of Alfalfa Seeds. J. Food Sci. 1979, 44, 443–445. [Google Scholar] [CrossRef]
- Fan, X.; Thayer, D.W.; Sokorai, K.J.B. Changes in Growth and Antioxidant Status of Alfalfa Sprouts during Sprouting as Affected by Gamma Irradiation of Seeds. J. Food Prot. 2004, 67, 561–566. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, B.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, X.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Lajter, I.; Zupkó, I.; Molnár, J.; Jakab, G.; Balogh, L.; Vasas, A.; Hohmann, J. Antiproliferative Activity of Polygonaceae Species from the Carpathian Basin against Human Cancer Cell Lines. Phytother. Res. 2013, 27, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Wang, Q.; Han, X.; Hao, J. Effect of pH and chlorine concentration of slightly acidic electrolyzed water on the buckwheat sprouts during germination. J. Food Process. Preserv. 2019, 43, e14175. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Watanabe, M.; Ito, M. Changes in Antioxidative Activity and Flavonoid Composition of the Extracts from Aerial Parts of Buckwheat during Growth Period. J. Jpn. Soc. Food Sci. Technol. 2002, 49, 119–125. [Google Scholar] [CrossRef]
- Margna, U.; Margna, E. Differential Biosynthesis of Buckwheat Flavonoids from Endogenous and Exogenous Substrates. Biochem. Physiol. Pflanz. 1978, 173, 347–354. [Google Scholar] [CrossRef]
- Ren, S.-C.; Sun, J.-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods 2014, 7, 298–304. [Google Scholar] [CrossRef]
- Jeong, H.; Sung, J.; Yang, J.; Kim, Y.; Jeong, H.S.; Lee, J. Effect of sucrose on the functional composition and antioxidant capacity of buckwheat (Fagopyrum esculentum M.) sprouts. J. Funct. Foods 2018, 43, 70–76. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Wang, W.; Liu, H. Dual effects of slightly acidic electrolyzed water (SAEW) treatment on the accumulation of γ-aminobutyric acid (GABA) and rutin in germinated buckwheat. Food Chem. 2016, 201, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xia, S.; Le, G.-W. Gamma-aminobutyric acid improves oxidative stress and function of the thyroid in high-fat diet fed mice. J. Funct. Foods 2014, 8, 76–86. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Charron, C.S.; Clevidence, B.A.; Britz, S.J.; Novotny, J.A. Effect of Dose Size on Bioavailability of Acylated and Nonacylated Anthocyanins from Red Cabbage (Brassica oleracea L. Var. capitata). J. Agric. Food Chem. 2007, 55, 5354–5362. [Google Scholar] [CrossRef]
- Jacob, J.A.; Mahal, H.; Mukherjee, T.; Kapoor, S. Free radical reactions with the extract of brassica family. Food Chem. 2011, 129, 1132–1138. [Google Scholar] [CrossRef]
- Podsędek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix Effects on the Stability and Antioxidant Activity of Red Cabbage Anthocyanins under Simulated Gastrointestinal Digestion. BioMed Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Devkota, H.P. Analysis of glucosinolates. In Recent Advances in Natural Products Analysis; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 651–661. [Google Scholar]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Peñas, E.; Frias, J.; Martínez-Villaluenga, C.; Vidal-Valverde, C. Bioactive Compounds, Myrosinase Activity, and Antioxidant Capacity of White Cabbages Grown in Different Locations of Spain. J. Agric. Food Chem. 2011, 59, 3772–3779. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef]
- Perez, C.; Barrientos, H.; Román, J.; Mahn, A. Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem. 2014, 145, 264–271. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts. Cancer Epidemiol. Prev. Biomark. 2001, 10, 501–508. [Google Scholar]
- Gu, Z.-X.; Guo, Q.-H.; Gu, Y.-J. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control, Prevention (US); National Immunization Program (Centers for Disease Control and Prevention). Epidemiology and Prevention of Vaccine-Preventable Diseases; Department of Health & Human Services, Public Health Service, Centers for Disease Control and Prevention: Washington, DC, USA, 2005.
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Domagała, D.; Kusznierewicz, B. Young shoots of red cabbage are a better source of selected nutrients and glucosinolates in comparison to the vegetable at full maturity. Eur. Food Res. Technol. 2020, 246, 2505–2515. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat. Int. J. Mol. Sci. 2010, 11, 5201–5211. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.B.; Anani, K.; Lee, M.K.; De Souza, C.; Arnason, J.T.; Gbeassor, M. Further Iinvestigations on the Antiviral Activities of Medicinal Plants of Togo. Pharm. Biol. 2000, 38, 46–50. [Google Scholar] [CrossRef]
- Gatouillat, G.; Magid, A.A.; Bertin, E.; Okiemy-Akeli, M.-G.; Morjani, H.; Lavaud, C.; Madoulet, C. Cytotoxicity and Apoptosis Induced by Alfalfa (Medicago sativa) Leaf Extracts in Sensitive and Multidrug-Resistant Tumor Cells. Nutr. Cancer 2014, 66, 483–491. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, T.; Wang, Y.; Li, Y.-T.; Gowd, V.; Niu, X.-H.; Yang, H.-Y.; Chen, L.-S.; Chen, W.; Sun, C.-D. Antioxidant and antidiabetic properties of tartary buckwheat rice flavonoids after in vitro digestion. J. Zhejiang Univ. Sci. B 2016, 17, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.C.; Babu, K.R.; Madhu, M.; Gopinath, C. In vitro Antidiabetic Activity of Sulforaphane. Pharmacol. Toxicol. Biomed. Rep. 2017, 3, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-M.; Arasu, M.V.; Kim, Y.-B.; Park, S.U.; Kim, S.-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 177, 204–213. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Ibrahim, H.S.; Ahmed, L.A. The Hypoglycemic and Anti-inflammatory Effects of Alfalfa (Medicago sativa L.). Plant Rats 2015, 31, 71–90. [Google Scholar]
- Baxi, D.B.; Singh, P.K.; Doshi, A.A.; Arya, S.; Mukherjee, R.; Ramachandran, A.V. Medicago sativa leaf extract supplementation corrects diabetes induced dyslipidemia, oxidative stress and hepatic renal functions and exerts antihyperglycaemic action as effective as metformin. Ann. Biol. Res. 2010, 1, 107–119. [Google Scholar]
- Yamagishi, S.-I. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets 2011, 12, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Sotokawauchi, A.; Ishibashi, Y.; Matsui, T.; Yamagishi, S.-I. Aqueous Extract of Glucoraphanin-Rich Broccoli Sprouts Inhibits Formation of Advanced Glycation End Products and Attenuates Inflammatory Reactions in Endothelial Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 9823141. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-Y.; Peng, C.-C.; Yang, Y.-L.; Peng, R.Y. Optimization of Bioactive Compounds in Buckwheat Sprouts and Their Effect on Blood Cholesterol in Hamsters. J. Agric. Food Chem. 2008, 56, 1216–1223. [Google Scholar] [CrossRef]
- Harwood, H.J.; Charles, E.C.; Lorraine, D.P.; Bangerter, F.W.; Wilkins, R.W.; Long, C.A.; Cosgrove, P.G.; Malinow, M.R.; Marzetta, C.A.; Pettini, J.L. Pharmacologic consequences of cholesterol absorption inhibition: Alteration in cholesterol metabolism and reduc-tion in plasma cholesterol concentration induced by the synthetic saponin beta-tigogenin cellobioside (CP-88818; tiqueside). J. Lipid Res. 1993, 34, 377–395. [Google Scholar] [CrossRef]
- Petit, P.R.; Sauvaire, Y.D.; Hillaire-Buys, D.M.; Leconte, O.M.; Baissac, Y.G.; Ponsin, G.R.; Ribes, G.R. Steroid saponins from fenugreek seeds: Extraction, purification, and pharmacological investigation on feeding behavior and plasma cholesterol. Steroids 1995, 60, 674–680. [Google Scholar] [CrossRef]
- Al-Habori, M.; Raman, A. Antidiabetic and hypocholesterolaemic effects of fenugreek. Phytother. Res. 1998, 12, 233–242. [Google Scholar] [CrossRef]
- Castro-Torres, I.G.; DE LA O-ARCINIEGA, M.; Martínez-Vázquez, M. Two glucosinolates and their effects related to the prevention of cholesterol gallstones: A Review. Bol. Latinoam. Caribe Plant. Med. Aromát. 2014, 13, 1–9. [Google Scholar]
- Neagu, I.A.; Olejarz, J.; Freeman, M.; Rosenbloom, D.I.; Nowak, M.A.; Hill, A.L. Life cycle synchronization is a viral drug resistance mechanism. PLoS Comput. Biol. 2018, 14, e1005947. [Google Scholar] [CrossRef] [Green Version]
- Kormuth, K.A.; Lakdawala, S.S. Emerging antiviral resistance. Nat. Microbiol. 2020, 5, 4–5. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Abdulamir, A.S.; Abu Bakar, F.; Sekawi, Z.; Jahansheri, F.; Jalilian, F.A. Novel antiviral activity of mung bean sprouts against respiratory syncytial virus and herpes simplex virus−1: An in vitro study on virally infected Vero and MRC-5 cell lines. BMC Complement. Altern. Med. 2015, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Noah, T.L.; Zhang, H.; Zhou, H.; Glista-Baker, E.; Müller, L.; Bauer, R.N.; Meyer, M.; Murphy, P.C.; Jones, S.; Letang, B.; et al. Effect of Broccoli Sprouts on Nasal Response to Live Attenuated Influenza Virus in Smokers: A Randomized, Double-Blind Study. PLoS ONE 2014, 9, e98671. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. The pathogenesis of atherosclerosis—An update. New Engl. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef]
- Malinow, M.; McLaughlin, P.; Stafford, S.; Livingston, A.; Kohler, G. Alfalfa saponins and alfalfa seeds: Dietary effects in cholesterol-fed rabbits. Atherosclerosis 1980, 37, 433–438. [Google Scholar] [CrossRef]
- A Story, J.; Lepage, S.L.; Petro, M.S.; West, L.G.; Cassidy, M.M.; Lightfoot, F.G.; Vahouny, G.V. Interactions of alfalfa plant and sprout saponins with cholesterol in vitro and in cholesterol-fed rats. Am. J. Clin. Nutr. 1984, 39, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.; Ghodke, M.S. Advances and Perspectives of Gamma-Aminobutyric Acid as a Bioactive Compound in Food. In Advanced Structured Materials; Springer Science and Business Media LLC: Berlin, Germany, 2021; pp. 819–843. [Google Scholar]
- Watanabe, M.; Ayugase, J. Anti-stress Effects of Flavonoids from Buckwheat Sprouts in Mice Subjected to Restraint Stress. Food Sci. Technol. Res. 2008, 14, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.R.; Pereira, M.J.; Azevedo, J.; Gonçalves, R.F.; Valentão, P.; de Pinho, P.G.; Andrade, P.B. Glycine max (L.) Merr., Vigna radiata L. and Medicago sativa L. sprouts: A natural source of bioactive compounds. Food Res. Int. 2013, 50, 167–175. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Shao, X.; Xu, F.; Wang, H. Sucrose treatment of mung bean seeds results in increased vitamin C, total phenolics, and antioxidant activity in mung bean sprouts. Food Sci. Nutr. 2019, 7, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Torres, E. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed. Pharmacother. 2003, 57, 251–260. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Wang, S.-C.; Hsu, C.; Lin, B.-F.; Kuo, Y.-H.; Huang, C.-J. Phytoestrogenic Compounds in Alfalfa Sprout (Medicago sativa) beyond Coumestrol. J. Agric. Food Chem. 2011, 59, 131–137. [Google Scholar] [CrossRef]
- Vale, A.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.; Oliveira, M.B.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, D.; He, X.; Nirasawa, S.; Tatsumi, E.; Liu, H. The relationship between antioxidant enzymes activity and mungbean sprouts growth during the germination of mungbean seeds treated by electrolyzed water. Plant Growth Regul. 2014, 74, 83–91. [Google Scholar] [CrossRef]
- Li, L.; Hao, J.; Song, S.; Nirasawa, S.; Jiang, Z.; Liu, H. Effect of slightly acidic electrolyzed water on bioactive compounds and morphology of broccoli sprouts. Food Res. Int. 2018, 105, 102–109. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Fan, Y.-F.; Ling, J.-G.; Hu, Y.; Liu, D.-H.; Chen, S.; Ye, X.-Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and antioxidant composition of quinoa (Chenopodium quinoa Willd.) sprout from seeds submitted to water stress, salinity and light conditions. Ind. Crop. Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Rui, L.; Jianxiong, H.; Haijie, L.; Lite, L. Application of electrolyzed functional water on producing mung bean sprouts. Food Control 2011, 22, 1311–1315. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Alfocea, F.P.; Hernandez, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef]
- Li, F.-J.; Cheng, Y.-Q.; Yin, L.-J.; Liu, H.-J.; Li, L.-T. Application of Electrolyzed Water to Improve Angiotensin I-Converting Enzyme Inhibitory Activities of Fermented Soybeans Started with Bacillus Subtilis B1. Int. J. Food Prop. 2011, 14, 145–156. [Google Scholar] [CrossRef]
- Hao, J.; Li, J.; Zhao, D. Effect of slightly acidic electrolysed water on functional components, antioxidant and α-glucosidase inhibitory ability of buckwheat sprouts. Int. J. Food Sci. Technol. 2021, 56, 3463–3473. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Li, Z.; Liu, Q.; Zhao, X.; Yang, H. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019, 286, 87–97. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Cui, J. Increased sucrose in the hypocotyls of radish sprouts contributes to nitrogen deficiency-induced anthocyanin accumulation. Front. Plant Sci. 2016, 7, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Tan, G.J.T.; Pang, X.; Yuan, W.; Lai, S.; Yang, H. Energy Regulated Nutritive and Antioxidant Properties during the Germination and Sprouting of Broccoli Sprouts (Brassica oleracea var. italica). J. Agric. Food Chem. 2018, 66, 6975–6985. [Google Scholar] [CrossRef]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of calcium chloride and sucrose on the composition of bioactive compounds and antioxidant activities in buckwheat sprouts. Food Chem. 2020, 312, 126075. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Shahinozzaman, M.; Tien, N.T.K.; Thach, T.N.; Tawata, S. Effect of sucrose on antioxidant activities and other health-related micronutrients in gamma-aminobutyric acid (GABA)-enriched sprouting Southern Vietnam brown rice. J. Cereal Sci. 2020, 93, 102985. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed] [Green Version]
- Lim, Y.J.; Jeong, H.Y.; Gil, C.S.; Kwon, S.-J.; Na, J.K.; Lee, C.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food Chem. 2020, 303, 125376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usita-tissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Xiang, N.; Guo, X.; Liu, F.; Li, Q.; Hu, J.; Brennan, C.S. Effect of Light- and Dark-Germination on the Phenolic Biosynthesis, Phytochemical Profiles, and Antioxidant Activities in Sweet Corn (Zea mays L.) Sprouts. Int. J. Mol. Sci. 2017, 18, 1246. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, Y.; Hu, T.; Zhang, S.; Zhang, Y.; Zhao, T.; Yu, H.; Kang, Y. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Walton, D.C. L-phenylalanine ammonia-lyase activity during germination of Phaseolus vulgaris. Plant Physiol. 1968, 43, 1120–1124. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Ouyang, C.; Wang, S.; Xu, Y.; Tang, L.; Chen, F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008, 54, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Koocheki, A.; Taherian, A.R.; Razavi, S.M.; Bostan, A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009, 23, 2369–2379. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2017, 53, 516–524. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface meth-odology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Optimization of germination time and temperature to maximize the content of bioactive compounds and the antioxidant activity of purple corn (Zea mays L.) by response surface methodology. LWT 2017, 76, 236–244. [Google Scholar] [CrossRef]
- Germains. What Is Seed Priming? 2016. Available online: https://germains.com/what-is-seed-priming/#:~:text=Seed%20priming%20is%20a%20process%20of%20regulating%20the,the%20seed%20closer%20to%20the%20point%20of%20germination (accessed on 8 January 2016).
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- Baenas, N.; Villaño, D.; Garcia-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef]
- Afzal, I.; Aslam, N.; Mahmood, F.; Hameed, A.; Irfan, S.; Ahmad, G. Enhancement of Germination and Emergence of Canola Seeds by Different Priming Techniques; University of Santa Cruz do Sul: Santa Cruz do Sul, Brazil, 2004. [Google Scholar]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Afzal, I.; Rauf, S.; Basra, S.; Murtaza, G. Halopriming improves vigor, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ. 2008, 54, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Foti, R.; Abureni, K.; Tigere, A.; Gotosa, J.; Gere, J. The efficacy of different seed priming osmotica on the establishment of maize (Zea mays L.) caryopses. J. Arid. Environ. 2008, 72, 1127–1130. [Google Scholar] [CrossRef]
- Cayuela, E.; Pérez-Alfocea, F.; Caro, M.; Bolarin, M.C. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol. Plant. 1996. 96, 231–236.
- Wei, Y.; Shohag, M.; Ying, F.; Yang, X.; Wu, C.; Wang, Y.; Shohag, J.I. Effect of ferrous sulfate fortification in germinated brown rice on seed iron concentration and bioavailability. Food Chem. 2013, 138, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Prom-U-Thai, C.; Fukai, S.; Godwin, I.; Rerkasem, B.; Huang, L. Iron-fortified parboiled rice—A novel solution to high iron density in rice-based diets. Food Chem. 2008, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-A.; Grusak, M.A.; Oh, M.-M. Concentrations of minerals and phenolic compounds in three edible sprout species treated with iron-chelates during imbibition. Hortic. Environ. Biotechnol. 2014, 55, 471–478. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazo-Vélez, M.A.; Avilés-González, J.; Serna-Saldivar, S.O.; Temblador-Pérez, M.C. Optimization of wheat sprouting for production of selenium enriched kernels using response surface method-ology and desirability function. LWT Food Sci. Technol. 2016, 65, 1080–1086. [Google Scholar] [CrossRef]
- Yin, R.; Ulm, R. How plants cope with UV-B: From perception to response. Curr. Opin. Plant Biol. 2017, 37, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- Supapvanich, S.; Anan, W.; Chimsonthorn, V. Efficiency of combinative salicylic acid and chitosan preharvest-treatment on antioxidant and phytochemicals of ready to eat daikon sprouts during storage. Food Chem. 2019, 284, 8–15. [Google Scholar] [CrossRef]
- Miao, H.-Y.; Wang, M.-Y.; Chang, J.-Q.; Tao, H.; Sun, B.; Wang, Q.-M. Effects of glucose and gibberellic acid on glucosinolate content and antioxidant properties of Chinese kale sprouts. J. Zhejiang Univ. Sci. B 2017, 18, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Meier, F.; Lo, J.A.; Yuan, W.; Sze, V.L.P.; Chung, H.-J.; Yuk, H.-G. Overview of Recent Events in the Microbiological Safety of Sprouts and New Intervention Technologies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 265–280. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Raw Produce: Selecting and Serving it Safely; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Butscher, D.; Van Loon, H.; Waskow, A.; von Rohr, P.R.; Schuppler, M. Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int. J. Food Microbiol. 2016, 238, 222–232. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated With Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Multistate Outbreak of Shiga Toxin-Producing Escherichia Coli O26 Infections Linked to Raw Clover Sprouts at Jimmy John’s Restaurants; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2012.
- Centers for Disease Control and Prevention. Division of Foodborne, Waterborne, and Environmental Diseases (DFWED); Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID): Atlanta, GA, USA, 2018.
- US Food and Drug Administration. FDA Investigates Multistate Outbreak of Salmonella Infections Linked to Alfalfa Sprouts; US Food and Drug Administration: Silver Spring, MD, USA, 2016.
- Millan-Sango, D.; Sammut, E.; Van Impe, J.F.; Valdramidis, V.P. Decontamination of alfalfa and mung bean sprouts by ultrasound and aqueous chlorine dioxide. LWT 2017, 78, 90–96. [Google Scholar] [CrossRef]
- Lund, D. Effects of Heat Processing on Nutrients, in Nutritional Evaluation of Food Processing; Springer: Berlin/Heidelberg, Germany, 1988; pp. 319–354. [Google Scholar]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radiofrequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.-W.; Mok, C. Effects of Nonthermal Plasma Treatment on Decontamination and Sprouting of Radish (Raphanus sativus L.) Seeds. Food Bioprocess Technol. 2017, 10, 1093–1102. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Gaunt, L.F.; Beggs, C.B.; Georghiou, G. Bactericidal Action of the Reactive Species Produced by Gas-Discharge Nonthermal Plasma at Atmospheric Pressure: A Review. IEEE Trans. Plasma Sci. 2006, 34, 1257–1269. [Google Scholar] [CrossRef]
- Oh, D.-H.; Khan, I.; Tango, C.N. Hurdle Enhancement of Electrolyzed Water with Other Techniques. In Electrolyzed Water in Food: Fundamentals and Applications; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 231–260. [Google Scholar]
- Puligundla, P.; Kim, J.W.; Mok, C. Broccoli sprout washing with electrolyzed water: Effects on microbiological and physico-chemical characteristics. LWT 2018, 92, 600–606. [Google Scholar] [CrossRef]
- José, J.S.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Neto, L.; Millan-Sango, D.; Brincat, J.-P.; Cunha, L.M.; Valdramidis, V.P. Impact of ultrasound decontamination on the microbial and sensory quality of fresh produce. Food Control 2019, 104, 262–268. [Google Scholar] [CrossRef]
- Ngnitcho, P.-F.K.; Khan, I.; Tango, C.N.; Hussain, M.S.; Oh, D.H. Inactivation of bacterial pathogens on lettuce, sprouts, and spinach using hurdle technology. Innov. Food Sci. Emerg. Technol. 2017, 43, 68–76. [Google Scholar] [CrossRef]
- Žudytė, B.; Lukšienė, Ž. Toward better microbial safety of wheat sprouts: Chlorophyllin-based photosensitization of seeds. Photochem. Photobiol. Sci. 2019, 18, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Ngnitcho, P.-F.K.; Tango, C.N.; Khan, I.; Daliri, E.B.M.; Chellian, R.; Oh, D.H. The applicability of Weibull model for the kinetics inactivation of Listeria monocytogenes and Escherichia coli O157: H7 on soybean sprouts submitted to chemical sanitizers in combination with ultrasound at mild temperatures. LWT 2018, 91, 573–579. [Google Scholar] [CrossRef]
- Lukseviciute, V.; Luksiene, Z. Inactivation of molds on the surface of wheat sprouts by chlorophyllin-chitosan coating in the presence of visible LED-based light. J. Photochem. Photobiol. B Biol. 2020, 202, 111721. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.; Bhunia, A. Sequential disinfection of Escherichia coli O157:H7 inoculated alfalfa seeds before and during sprouting using aqueous chlorine dioxide, ozonated water, and thyme essential oil. LWT 2003, 36, 235–243. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Waje, C.; Jun, S.; Lee, Y.; Kim, B.; Han, D.; Jo, C.; Kwon, J. Microbial quality assessment and pathogen inactivation by electron beam and gamma irradiation of commercial seed sprouts. Food Control 2009, 20, 200–204. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.-H. Hurdle technology: A novel approach for enhanced food quality and safety—A Review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- National Advisory Committee on Microbiological Criteria for Foods. Microbiological safety evaluations and recommendations on fresh produce. Food Control 1999, 10, 117–143. [Google Scholar] [CrossRef]
- Thompson, S.; Powell, D.A. Risks associated with the consumption of fresh sprouts. Food Saf. Netw. Tech. Rep. 2000, 2000, 16. [Google Scholar]
- Mortimore, S.; Wallace, C. HACCP: A Practical Approach; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- De Oliveira, C.A.F.; Da Cruz, A.G.; Tavolaro, P.; Corassin, C.H. Food Safety: Good Manufacturing Practices (GMP), Sanitation Standard Operating Procedures (SSOP), Hazard Analysis and Critical Control Point (HACCP), in Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 129–139. [Google Scholar]
- International Sprout Growers Association. International Sprout Growers Association (ISGA): Warwick, RI, USA, 1989. Available online: https://isga-sprouts.org/ (accessed on 4 April 2021).
- Choi, Y.H.; Jiménez, G.P. Biotransformation of Plant Metabolites in Microorganisms. 2017. Available online: https://www.universiteitleiden.nl/en/research/research-projects/science/ibl-biotransformation-of-plant-metabolites-in-microorgan-isms#:~:text=Plant%20metabolites%20are%20biontransformed%20by%20enzymes%2C%20bacteria%20or,of%20metabolites%20and%20deconvoluted%20by%20metabolomic%20profiling%20techniques (accessed on 4 April 2021).
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A.A. Current approaches for enhanced expression of secondary metabolites as bioactive compounds in plants for agronomic and human health purposes—A review. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Fica, V.; Olteanu, D.; Oprescu, S. Use of spirulina as an adjuvant nutrient factor in the therapy of the diseases accompanying a nutritional deficiency (preliminary note). Rev. Med. Interna Neurol. Psihiatr. Neurochir. Dermato-Venerologie. Med. Interna 1984, 36, 225–232. [Google Scholar]
- Panjiar, N.; Mishra, S.; Yadav, A.N.; Verma, P. Functional foods from cyanobacteria: An emerging source for functional food products of pharmaceutical importance. Microb. Funct. Foods Nutraceuticals 2017, 2017, 21–37. [Google Scholar]
- Chen, T.; Piao, M.; Rahman, S.M.E.; Zhang, L.; Deng, Y. Influence of fermentation on antioxidant and hypolipidemic properties of maifanite mineral water-cultured common buckwheat sprouts. Food Chem. 2020, 321, 126741. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and Growth of Au Nanoparticles inside Live Alfalfa Plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Park, S.; Sung, H.K.; Kim, Y. Green Synthesis of Metal Nanoparticles Using Sprout Plants: Pros and Cons. J. Nanosci. Nanotechnol. 2016, 16, 4444–4449. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jribi, S.; Antal, O.T.; Fustos, Z.; Papai, G.; Naar, Z.; Kheriji, O.; Debbabi, H. Influence of sprouting bioprocess on durum wheat (Triticum durum) prebiotic properties. In Proceedings of the Research and innovation as tools for sustainable agriculture, food and nutrition security, Bari, Italy, 18–20 September 2018. [Google Scholar]
- Chen, Y.; Chang, S.K.; Zhang, Y.; Hsu, C.-Y.; Nannapaneni, R. Gut microbiota and short chain fatty acid composition as affected by legume type and processing methods as assessed by simulated in vitro digestion assays. Food Chem. 2020, 312, 126040. [Google Scholar] [CrossRef]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef]
- Rose, D.J.; Poudel, R.; Van Haute, M.J.; Yang, Q.; Wang, L.; Singh, M.; Liu, S. Pulse processing affects gas production by gut bacteria during in vitro fecal fermentation. Food Res. Int. 2021, 147, 110453. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Hou, G.G.; Cardin, M.; Marquart, L.; Dubat, A. Quality attributes of whole-wheat flour tortillas with sprouted whole-wheat flour substitution. LWT 2017, 77, 1–7. [Google Scholar] [CrossRef]
- Finnie, S.; Brovelli, V.; Nelson, D. Sprouted Grains as a Food Ingredient Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–142. [Google Scholar]
- Lorenz, K.; D’Appolonia, B. Cereal sprouts: Composition, nutritive value, food applications. Crit. Rev. Food Sci. Nutr. 1980, 13, 353–385. [Google Scholar] [CrossRef]
- Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Study of Active Ingredients in Black Soybean Sprouts and Their Safety in Cosmetic Use. Molecules 2012, 17, 11669–11679. [Google Scholar] [CrossRef] [PubMed]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; No, H.K. Composite wheat-germinated brown rice flours: Selected physicochemical properties and bread application. Int. J. Food Sci. Technol. 2011, 47, 75–82. [Google Scholar] [CrossRef]
- Phattanakulkaewmorie, N.; Paseephol, T.; Moongngarm, A. Chemical compositions and physico-chemical properties of malted sorghum flour and characteristics of gluten free bread. World Acad. Sci. Eng. Technol. 2011, 5, 532–538. [Google Scholar]
- Chung, H.-J.; Cho, A.; Lim, S.-T. Effect of heat-moisture treatment for utilization of germinated brown rice in wheat noodle. LWT 2012, 47, 342–347. [Google Scholar] [CrossRef]
- E Adedeji, O.; Oyinloye, O.D.; Ocheme, O.B. Effects of germination time on the functional properties of maize flour and the degree of gelatinization of its cookies. Afr. J. Food Sci. 2014, 8, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Mäkinen, O.; Zannini, E.; Arendt, E.K. Germination of Oat and Quinoa and Evaluation of the Malts as Gluten Free Baking Ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zagorska, J.; Rakcejeva, T.; Liepa, M. Quality characteristics of yoghurt enriched with wheat grain flakes. In Proceedings of the 1st UK International Functional Food Conference, Oxford, UK, 24 November 2010. [Google Scholar]
- Wu, F.; Xu, X. Sprouted Grains-Based Fermented Products Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–173. [Google Scholar]
- Klose, C.; Mauch, A.; Wunderlich, S.; Thiele, F.; Zarnkow, M.; Jacob, F.; Arendt, E.K. Brewing with 100% Oat Malt. J. Inst. Brew. 2011, 117, 411–421. [Google Scholar] [CrossRef]
- Choi, U.-K.; Jeong, Y.-S.; Kwon, O.-J.; Park, J.-D.; Kim, Y.-C. Comparative Study of Quality Characteristics of Korean Soy Sauce Made with Soybeans Germinated Under Dark and Light Conditions. Int. J. Mol. Sci. 2011, 12, 8105–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J. Food Sci. Technol. 2014, 52, 5129–5137. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Frias, J.; Granito, M.; Vidal-Valverde, C. Germinated Cajanus cajan seeds as ingredients in pasta prod-ucts: Chemical, biological and sensory evaluation. Food Chem. 2007, 101, 202–211. [Google Scholar] [CrossRef]
- Mattioli, S.; Bosco, A.D.; Martino, M.; Ruggeri, S.; Marconi, O.; Sileoni, V.; Falcinelli, B.; Castellini, C.; Benincasa, P. Alfalfa and flax sprouts supplementation enriches the content of bioactive compounds and lowers the cholesterol in hen egg. J. Funct. Foods 2016, 22, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Merendino, N.; Molinari, R.; Costantini, L.; Mazzucato, A.; Pucci, A.; Bonafaccia, F.; Bonafaccia, G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014, 5, 1017–1026. [Google Scholar] [CrossRef]
- Sudha, A.; Devi, K.; Sangeetha, V.; Sangeetha, A. Development of Fermented Millet Sprout Milk Beverage Based on Physicochemical Property Studies and Consumer Acceptability Data; NISCAIR-CSIR: New Delhi, India, 2016. [Google Scholar]



| Plant Sprouts | Bioactive Compounds | Health Benefits | Reference |
|---|---|---|---|
| Alfalfa | Saponins | Anticancer and antimicrobial activities | [23,24] |
| Flavonoids | Anti-inflammatory, antioxidant, antidiabetic activities | [77] | |
| Phenolic acids (ferulic, garlic, and caffeic acids) | Anti-inflammatory, antioxidant, antidiabetic activities | [77] | |
| Vitamins C and E and β-carotene | Antioxidant and anti-obesity activities | [20,78,79] | |
| Trace elements (copper, manganese, selenium) | Antidiabetic and antioxidant activities enhance functions of enzymes | [18,80] | |
| Coumestrol | Anti-obesity | [81] | |
| Buckwheat | Flavonoids (orientin, vitexin, rutin, and their derivatives) Quercetin, lectins | Anti-inflammatory, hypocholesterolemic, antioxidant, antidiabetic, and anticancer activities | [7,76] |
| anthocyanins | Antioxidant and antidiabetic activities | [29] | |
| 2″-hydroxynicotianamine | Antihypertension | [7] | |
| Aminobutyric acid | Antistress and antioxidant activities | [26,34] | |
| Red cabbage and broccoli | Organic acids (ascorbic acid, aconitic acid, shikimic acid, citric acid, oxalic acid, etc.) | Antibacterial, antioxidant activities | [82,83] |
| Glucosinolates (4-methylsulfinylbutyl isothiocyanate) | Anticancer, anti-AGE, hypocholesterolemic, anti-obesity activities | [20,40,45,79] | |
| Gallic, chlorogenic, sinapinic, benzoic, and ferulic acids, kaempferol | Anti-inflammatory, hypocholesterolemic, antioxidant activities | [39] | |
| Anthocyanin | Anticancer, antioxidant, anti-inflammatory activities | [35,36] |
| Bacteria | Year | Prevalence | Source (Sprouts) | Country | Reference |
|---|---|---|---|---|---|
| E. coli O103 | 2020 | 51 | Clover sprouts | USA | https://www.cdc.gov/ecoli/2020/o103h2-02-20/index.html. Accessed date (12 April 2021) |
| Salmonella | 2016 | 26 | Alfalfa | USA | [132] |
| E. coli O121 | 2014 | 19 | Alfalfa | USA | [130] |
| E. coli O26 | 2012 | 29 | Raw clover | USA | [131] |
| E. coliO104:H4 | 2011 | 3842 | Fenugreek | Germany | [127] |
| Salmonella | 2010 | 190 | Bean sprouts | UK | [127] |
| Sprout | Product Added | Function | Reference |
|---|---|---|---|
| Wheat | Breadmaking | Modify pasting characteristics | [176] |
| Soybean | Cosmetics | Whitening agent | [177] |
| Wheat | Tortillas | Enhance shelf life and sensory attributes | [174] |
| Brown rice | bread | Improve textural properties | [178] |
| Sorghum | Bread | Soften the dough | [179] |
| Brown rice | noodels | Improves quality properties | [180] |
| Maize | Cookies | Modify gelatinization properties | [181] |
| Quinoa and oat | Bread | Improve the nutritional value | [182] |
| Wheat | Yoghurt | Improve a broad range of quality characteristics | [183] |
| Barley | Beer brewing | Improve the beer’s flavor, taste, and nutritional value | [184] |
| Oat | Beer brewing | Improve the aroma | [185] |
| Soybean, brown rice | Steamed buns | Enhance overall nutritional quality | [184] |
| Brown rice | Wine | Improve organoleptic properties | [184] |
| Barley, rice | Vinegar | Enhance the enzymatic activity and aroma | [184] |
| Soybean | Soy sauce | Improve organoleptic properties | [186] |
| Millet, soybean | Biscuits | Improve quality parameters (hardness, stickiness) | [187] |
| Pigeon pea | Semolina pasta | Increase the nutritional value | [188] |
| Alfalfa and flax | Hen egg | Enrichment lowering the cholesterol | [189] |
| Buckwheat | Pastor | Improve functionality | [190] |
| Millet | Milk beverage | Enhance the nutritional value | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible Plant Sprouts: Health Benefits, Trends, and Opportunities for Novel Exploration. Nutrients 2021, 13, 2882. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082882
Aloo SO, Ofosu FK, Kilonzi SM, Shabbir U, Oh DH. Edible Plant Sprouts: Health Benefits, Trends, and Opportunities for Novel Exploration. Nutrients. 2021; 13(8):2882. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082882
Chicago/Turabian StyleAloo, Simon Okomo, Fred Kwame Ofosu, Sheila M. Kilonzi, Umair Shabbir, and Deog Hwan Oh. 2021. "Edible Plant Sprouts: Health Benefits, Trends, and Opportunities for Novel Exploration" Nutrients 13, no. 8: 2882. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082882