Breastfeeding in Cystic Fibrosis: A Systematic Review on Prevalence and Potential Benefits
Abstract
:1. Introduction
2. Materials and Methods
3. Results
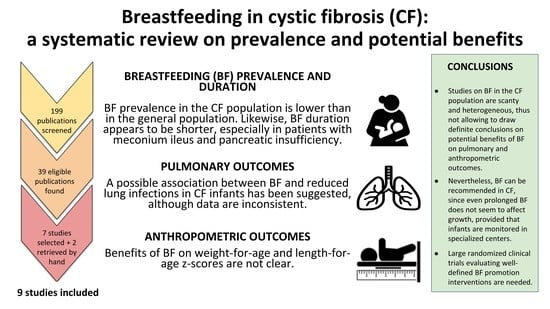
3.1. Selected Articles
3.2. Prevalence and Duration of Breastfeeding
3.3. Breastfeeding and Anthropometric Outcomes
3.4. Breastfeeding and Pulmonary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.A. Hypoproteinemia and anemia in infants with cystic fibrosis. A presenting symptom complex often misdiagnosed. JAMA J. Am. Med. Assoc. 1974, 228, 585–588. [Google Scholar] [CrossRef]
- Sokol, R.J.; Reardon, M.C.; Accurso, F.J.; Stall, C.; Narkewicz, M.; Abman, S.H.; Hammond, K.B. Fat-soluble-vitamin status during the first year of life in infants with cystic fibrosis identified by screening of newborns. Am. J. Clin. Nutr. 1989, 50, 1064–1071. [Google Scholar] [CrossRef]
- Borowitz, D.; Baker, R.D.; Stallings, V.; Bachrach, L.K.; Beall, R.J.; Ph, D.; Campbell, P.W.; Casey, S.C.; Cohen, M.B.; Corey, M.; et al. Consensus report on nutrition for pediatric patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- WHO. Exclusive Breastfeeding for Six Months Best for Babies Everywhere. Available online: http://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/ (accessed on 27 January 2021).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’connell, D.; Petersen, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Department of Epidemiology and Community Medicine, University of Ottawa: Ottawa, ON, Canada, 2012; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 January 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Smith, C. Supporting Optimal Growth in Infants with Chronic Conditions: How Are We Doing and What Can We Do? Breastfeed. Med. 2019, 14, S18–S19. [Google Scholar] [CrossRef] [Green Version]
- Luder, E.; Kattan, M.; Tanzer-Torres, G.; Bonforte, R.J. Current Recommendations for Breast-feeding in Cystic Fibrosis Centers. Am. J. Dis. Child. 1990, 144, 1153–1156. [Google Scholar] [CrossRef]
- Miller, T.; Antos, N.J.; Brock, L.A.; Wade, T.; Goday, P.S. Lactation Consultation Sustains Breast Milk Intake in Infants with Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 358–362. [Google Scholar] [CrossRef]
- Parker, E.M.; O’Sullivan, B.P.; Shea, J.C.; Regan, M.M.; Freedman, S.D. Survey of Breast-Feeding Practices and Outcomes in the Cystic Fibrosis Population. Pediatr. Pulmonol. 2004, 37, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Costantini, D.; Zazzeron, L.; Faelli, N.; Russo, M.C.; Ghisleni, D.; Gatelli, I.; Giovannini, M.; Riva, E.; Zetterström, R.; et al. Benefits of breastfeeding in cystic fibrosis: A single-centre follow-up survey. Acta Paediatr. 2007, 96, 1228–1232. [Google Scholar] [CrossRef]
- Jadin, S.A.; Wu, G.S.; Zhang, Z.; Shoff, S.M.; Tippets, B.M.; Farrell, P.M.; Miller, T.; Rock, M.J.; Levy, H.; Lai, H.J. Growth and pulmonary outcomes during the first 2 y of life of breastfed and formula-fed infants diagnosed with cystic fibrosis through the Wisconsin Routine Newborn Screening Program. Am. J. Clin. Nutr. 2011, 93, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, M.; Emerson, J.; McNamara, S.; Thompson, V.; Ramsey, B.W.; Morgan, W.; Gibson, R.L. Risk factors for age at initial Pseudomonas acquisition in the cystic fibrosis epic observational cohort. J. Cyst. Fibros. 2012, 11, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.H.; Heltshe, S.L.; Borowitz, D.; Gelfond, D.; Kloster, M.; Heubi, J.E.; Stalvey, M.; Ramsey, B.W.; Stecenko, A.; Schechter, M.; et al. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. JAMA Pediatr. 2017, 171, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Boulkedid, R.; Weiss, L.; Foucaud, P.; Wizla-Derambure, N.; Reix, P.; Bremont, F.; Derelle, J.; Schroedt, J.; Alberti, C. Nutritional Status in the First 2 Years of Life in Cystic Fibrosis Diagnosed by Newborn Screening. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 123–130. [Google Scholar] [CrossRef]
- Padoan, R.; Cirilli, N.; Falchetti, D.; Cesana, B.M. Risk factors for adverse outcome in infancy in meconium ileus cystic fibrosis infants: A multicentre Italian study. J. Cyst. Fibros. 2019, 18, 863–868. [Google Scholar] [CrossRef]
- Holliday, K.E.; Allen, J.R.; Waters, D.L.; Gruca, M.A.; Thompson, S.M.; Gaskin, K.J. Growth of human milk-fed and formula-fed infants with cystic fibrosis. J. Pediatr. 1991, 118, 77–79. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Linnane, B.; George, S.; Ni Chroinin, M.; Mullane, D.; Herzig, M.; Greally, P.; Elnazir, B.; Healy, F.; Mc Nally, P.; et al. Neonatal screening programme for CF: Results from the Irish Comparative Outcomes Study (ICOS). Pediatr. Pulmonol. 2020, 55, 2323–2329. [Google Scholar] [CrossRef]
- Brown, C.R.L.; Dodds, L.; Legge, A.; Bryanton, J.; Semenic, S. Factors influencing the reasons why mothers stop breastfeeding. Can. J. Public Health 2014, 105. [Google Scholar] [CrossRef]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.; Lönnerdal, B.; Abrams, S.A.; Hernell, O. Iron absorption in breast-fed infants: Effects of age, iron status, iron supplements, and complementary foods. Am. J. Clin. Nutr. 2002, 76, 198–204. [Google Scholar] [CrossRef]
- Lönnerdal, B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Proc. Am. J. Clin. Nutr. 2017, 106, 1681S–1687S. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| First Author (Year of Publication), Country [Reference] | Study Design | Enrolled Patients |
|---|---|---|
| Holliday et al. (1991), Australia [20] | Single-center retrospective cohort study | 65 CF infants without MI and 16 infants with MI |
| Parker EM et al. (2004), USA [13] | Multicenter survey | 768 CF patients |
| Colombo et al. (2007), Italy [14] | Single-center retrospective cohort study | 146 patients (aged 5–18 years) seen at the CF center between September 2003 and April 2004 |
| Jadin et al. (2011), USA [15] | Multicenter retrospective cohort study | 103 CF infants born between 1994 and 2006 who were diagnosed through NBS |
| Rosenfeld et al. (2012), USA [16] | Multicenter retrospective cohort study | 264 CF patients aged < 2 years with no isolation of P. aeruginosa from any respiratory culture prior to enrollment |
| Leung et al. (2017), USA [17] | Multicenter prospective cohort study | 231 CF infants younger than 3.5 months diagnosed by NBS |
| Munck et al. (2018), France [18] | Multicenter prospective cohort study | 105 infants with no history of MI recruited between April 2010 and September 2011 after the first visit for confirmatory CF diagnosis after NBS |
| Padoan et al. (2019), Italy [19] | Multicenter case–control study within a retrospective cohort study | 85 CF infants with MI born between 2009 and 2016 and diagnosed by symptoms |
| Fitzgerald et al. (2020), Ireland [21] | Multicenter retrospective cohort study | 186 CF infants (77 born between July 2008 and June 2011 clinically diagnosed and 109 born between July 2011 and June 2016 diagnosed through NBS) |
| First Author (Year of Publication) [Reference] | Study Design | Selection | Comparability | Exposure a | Outcome a | Score (Maximum: 9) |
|---|---|---|---|---|---|---|
| Holliday et al. (1991) [20] | Cohort | 4 | 0 | NA | 3 | 7 |
| Colombo et al. (2007) [14] | Cohort | 4 | 2 | NA | 3 | 9 |
| Jadin et al. (2011) [15] | Cohort | 4 | 1 | NA | 3 | 8 |
| Rosenfeld et al. (2012) [16] | Cohort | 4 | 0 | NA | 3 | 7 |
| Leung et al. (2017) [17] | Cohort | 4 | 0 | NA | 3 | 7 |
| Munck et al. (2018) [18] | Cohort | 4 | 1 | NA | 3 | 8 |
| Padoan et al. (2019) [19] | Case/control within a cohort | 4 | 0 | 3 | NA | 7 |
| Fitzgerald et al. (2020) [21] | Cohort | 4 | 2 | NA | 3 | 9 |
| First Author (Year of Publication) [Reference] | Prevalence of Breastfeeding |
|---|---|
| Holliday et al. (1991) [20] | Patients without MI: 63.1% BF at least 3 m, 46.2% exBF. Patients with MI: 43.8% BF at least 3 m. |
| Parker EM et al. (2004) [13] | 49% BF at any time, 18% exBF. |
| Colombo et al. (2007) [14] | 62% BF at any time, 23% BF > 4 m. BF rate at any time higher among PS patients (74%) than PI patients (57%). |
| Jadin et al. (2011) [15] | 51% BF at any time (26% exBF < 1 m, 10.7% ExBF = 1 m and 13.6% exBF ≥ 2 m). BF rates at any time higher among PS patients (66%) than PI patients without MI (48%) and patients with MI (54%). |
| Rosenfeld et al. (2012) [16] | Ever BF: 52.3%. |
| Leung et al. (2017), USA [17] | BF at 3 m of age: 43.3%, 25% exBF. |
| Munck et al. (2018) [18] | At initial visit the rates of exclusively or partial BF were 47% among PI patients and 20% among PS patients. Corresponding figures at 6 m: 20% among PI patients and 28% among PS patients. 47.6% of patients received exclusive BF during the first 3 m of life. |
| Padoan et al. (2019) [19] | Ever BF in the first 3 m of life: 54%. |
| Fitzgerald et al. (2020) [21] | Ever BF: 51.4% among the NBS group and 54.2% among the clinically diagnosed group. |
| First Author (Year of Publication) [Reference] | Comparisons | Length/Height | Weight/BMI |
|---|---|---|---|
| Holliday et al. (1991) [20] | BF ≥ 3 m vs. no BF or BF< 3 m | No significant differences in Lz at the age of 3, 6, 12, 18 and 24 m among infants without MI. In the subgroup of infants with MI, mean values of Lz at the age of 24 m were higher among infants BF ≥ 3 m as compared to those not BF or BF < 3 m: Lz at 24 m: 0.94 vs. −0.85, p = 0.01. | No significant differences in Wz at the age of 3, 6, 12, 18 and 24 m among infants without MI. In the subgroup of infants with MI, mean values of Wz at the age of 12 and 24 m were higher among infants BF ≥ 3 m as compared to those not BF or BF < 3 m: Wz at 12 m: 0.96 vs. −1.19, p = 0.001, Wz at 24 m: 0.94 vs. −0.85, p = 0.007. |
| Parker EM et al. (2004) [13] | noBF, BF supplemented with formula <6 m, BF supplemented with formula ≥ 6 m, exBF <6 m, exBF ≥ 6 m | Not evaluated | No significant differences in BMI (p = 0.67) |
| Colombo et al. (2007) [14] | BF > 4 m vs. no BF or 1–4 m BF (any type of BF: exclusive, predominant, partial) | No significant differences in Lz (at the time of enrollment and at 1 year of age). Mean Lz values at 1 year of age were −0.84, −1.03 and −0.70 among no BF, BF 1–4 m and BF > 4 m infants, respectively (p = 0.47). | No significant differences in Wz (at the time of enrollment and at 1 year of age). Mean Wz values at 1 year of age were −0.51, −0.68 and −0.44 among no BF, BF no BF, BF 1–4 m and BF > 4 m infants, respectively (p = 0.54). |
| Jadin et al. (2011) [15] | exBF < 1 m, 1 m, ≥ 2 m and exclusive standard caloric density formula (exFF20, 20 kcal per ounce) and high caloric density formula (exFF22, 22 kcal per ounce) | No significant differences in Lz. | Rapid decline in Wz from birth to 6 m of age observed only in children with exBF > 2 m (p < 0.0001). |
| Leung et al. (2017) [17] | exBF at 3 m of age vs. exFF or combination | Not evaluated | Wz at 3 m of age higher among exBF infants as compared to exFF and those receiving a combination of FF and BF (mean difference: 0.54, 95% CI: 0.22 to 0.87). However, no differences emerged when exBF infants were compared with exFF infants (mean difference at 3 m of age: 0.13, 95% CI: −0.17 to 0.44). No significant differences at 6 and 12 m of age. |
| Munck et al. (2018) [18] | Different comparisons for nutritional outcomes (exBF vs. exFF during the first 3 m of life) and pulmonary outcomes (exclusively or partially BF for 3 m vs. exFF) | No significant association between exBF and Lz ≥ 10th at the age of 2 years (OR: 0.66, 95% CI: 0.22–1.96). | No significant association between exBF and Wz ≥ 10th percentile (OR: 0.39, 95% CI: 0.08–1.99). |
| First Author (Year of Publication) [Reference] | Comparisons | Respiratory Function | Lung Infections and Other Pulmonary Outcomes |
|---|---|---|---|
| Parker EM et al. (2004) [13] | noBF, BF supplemented with formula <6 m, BF supplemented with formula ≥ 6 m, exBF <6 m, exBF ≥ 6 m | No significant differences in FEV1% collected at the time of filling out the survey questionnaire (p = 0.42). FEV1 values were ≤70%, 71-90% and >90% in 20, 33 and 47% of patients who were not BF as compared to 16, 31 and 55% of patients who were exBF ≥ 6 m. | Percentages of patients who received 0–1, 2–3, ≥4 courses of IV antibiotics over the 2 years prior to the enrolment were 72, 16 and 12% among no BF and 84, 10 and 6% among those exBF ≥ 6 m (p = 0.03). No significant differences in age at onset of symptoms (p = 0.28): 64, 75, 82 and 90% of patients who were not BF had symptoms onset by 3, 6, 12 and 24 m, respectively, as compared to 60, 72, 79 and 87% among patients who were exBF ≥ 6 m. |
| Colombo et al. (2007) [14] | BF > 4 m vs. no BF or 1–4 m BF (any type of BF: exclusive, predominant, or partial) | Mean FEV1% values at the time of enrollment were 91, 98 and 112 among no BF, BF 1–4 m and BF > 4 m infants, respectively (p < 0.001). Mean FVC% values at the time of enrollment were 91, 98 and 111 among no BF, BF 1–4 m and BF > 4 m infants, respectively (p < 0.001). | Mean number of infections during the first 3 years of life was: 5 among patients with BF > 4 m, 7.5 among those with BF 1–4 m and 8 in the no BF group (p = 0.015). No significant differences in number of hospitalizations during the first 3 years of life. |
| Jadin et al. (2011) [15] | ExBF < 1 m, 1 m, ≥ 2 m and exclusive standard caloric density formula (exFF20, 20 kcal per ounce) and high caloric density formula (exFF22, 22 kcal per ounce) | Not evaluated | Percentage of never colonized with P. aeruginosa through the first 2 years of age higher among infants with exBF = 1 m (90%) as compared to exBF < 1 m (44%), exBF ≥ 2 m (40%), exFF20 (43%) and exFF22 (50%). Percentage of patients who had ≥ 2 P. aeruginosa infections through the first 2 years of age lower among exBF = 1 m (0) and exBF ≥ 2 m (0) as compared to BF < 1 m (13%), exFF20 (32%) and exFF22 (43%) (p = 0.026 for all group comparison and p = 0.003 for BF vs. all FF infants). Mean values of Wisconsin CXR scores at the age of 2 year lower among exBF = 1 m (2.0) as compared to exBF < 1 m (4.5), exBF ≥ 2 m (5.7), exFF20 (3.4) and exFF22 (4.1) (p = 0.015). No significant differences in S. aureus infections. |
| Rosenfeld et al. (2012) [16] | BF in infancy vs. no BF | Not evaluated | No significant association between BF and P. aeruginosa acquisition (HR: 0.85, 95% CI: 0.60–1.21) |
| Munck et al. (2018) [18] | Different comparisons for nutritional outcomes (exBF vs. exFF during the first 3 m of life) and pulmonary outcomes (exclusively or partially BF for 3 m vs. exFF) | Not evaluated |
No significant differences in P. aeruginosa acquisition (37% among exFF infants and 23% among exclusively or partially BF infants, p = 0.10). Initial acquisition of P. aeruginosa> 1 year (25% among exFF infants and 64% among exclusively or partial BF infants, p = 0.06) |
| Fitzgerald et al. (2020) [21] | BF of variable duration vs. never BF | Not evaluated | No significant association between BF and hospitalization for pulmonary exacerbation in the first 36 m of life (OR adjusted for type of diagnosis, sex, sibling with CF, parent smoking, private health insurance and genotype: 1.85, 95% CI: 0.84–4.07.) |
| Padoan et al. (2019) [19] | BF of variable duration vs. never BF | Not evaluated | The risk of negative outcome as defined by chronic P. aeruginosa infection and/or faltering growth at 1 year of age was higher among never as compared to BF infants (OR 2.92, p = 0.061) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, C.; Alicandro, G.; Daccò, V.; Consales, A.; Mosca, F.; Agostoni, C.; Giannì, M.L. Breastfeeding in Cystic Fibrosis: A Systematic Review on Prevalence and Potential Benefits. Nutrients 2021, 13, 3263. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093263
Colombo C, Alicandro G, Daccò V, Consales A, Mosca F, Agostoni C, Giannì ML. Breastfeeding in Cystic Fibrosis: A Systematic Review on Prevalence and Potential Benefits. Nutrients. 2021; 13(9):3263. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093263
Chicago/Turabian StyleColombo, Carla, Gianfranco Alicandro, Valeria Daccò, Alessandra Consales, Fabio Mosca, Carlo Agostoni, and Maria Lorella Giannì. 2021. "Breastfeeding in Cystic Fibrosis: A Systematic Review on Prevalence and Potential Benefits" Nutrients 13, no. 9: 3263. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093263