Inhibition of Rotavirus Infectivity by a Neoglycolipid Receptor Mimetic
Abstract
:1. Introduction
2. Experimental Section
2.1. Cells and Virus
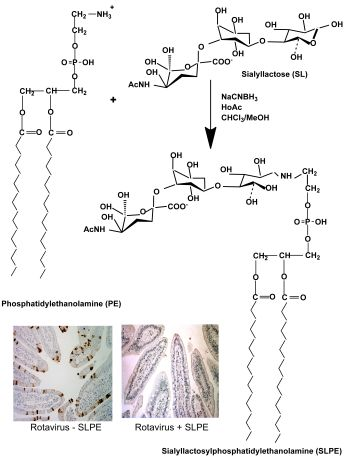
2.2. Synthesis of Neoglycolipids
2.3. Purification of Neoglycolipids by Preparative HPLC
2.4. Neoglycolipid Product Characterization
2.5. Synthesis of Sialyllactosyl-BSA
2.6. Acetylation of PE and PE Derivatives
2.7. Virus Radiolabeling and Host Cell Binding Assay
2.8. Virus-Glycolipid Binding Assay
2.9. Measurement of in Vitro Virus Infectivity: Focus-Forming Assay
2.10. Animal Infectivity Experiments
3. Results and Discussion
3.1. Synthesis, Purification, and Characterization of Sialyllactosylphoshatidylethanolamine




3.2. Rotavirus Binding to SLPE and LPE Neoglycolipids
3.3. SLPE Inhibits both Rotavirus Binding and Infectivity of Host Cells in Vitro

 ), LPE; (
), LPE; (  ), SLPE; (
), SLPE; (  ), NGcGM3; (
), NGcGM3; (  ), Sialyllactose-BSA (SL-BSA). SL-BSA concentrations are based on µM equivalent of sialic acid.
), Sialyllactose-BSA (SL-BSA). SL-BSA concentrations are based on µM equivalent of sialic acid.
 ), LPE; (
), LPE; (  ), SLPE; (
), SLPE; (  ), NGcGM3; (
), NGcGM3; (  ), Sialyllactose-BSA (SL-BSA). SL-BSA concentrations are based on µM equivalent of sialic acid.
), Sialyllactose-BSA (SL-BSA). SL-BSA concentrations are based on µM equivalent of sialic acid. 
3.4. SLPE Inhibits Rotavirus Infection and Disease in Piglets
| Piglets | Virus dose (FFU) | SLPE (µmol) | ELISA 40 h PI (% control) | ELISA 64 h PI | ELISA 88 h PI | Diarrhea |
|---|---|---|---|---|---|---|
| 1 | 1 × 105 | - | 2.704 (100) | 0.938 | 1.449 | + |
| 2 | 1 × 105 | 1.0 | 0.931 (34) | 0.298 | 0 | − |
| 3 | 1 × 104 | - | 2.684 (100) | 1.026 | 0.218 | + |
| 4 | 1 × 104 | 1.0 | 0.845 (31) | 0 | 1.683 | −, + (88 h) |
| 5 | 1 × 103 | - | 2.520 (100) | 0 | 0.009 | + |
| 6 | 1 × 103 | 1.0 | 0.390 (15) | 0 | 1.358 | −, + (88 h) |



4. Conclusions
Acknowledgements
References
- Parashar, U.D.; Gibson, C.J.; Bresse, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg.Infect. Dis. 2006, 12, 304–306. [Google Scholar]
- Ciarlet, M.; Schodel, F. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine 2009, 27 (Suppl. 6), G72–G81. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Patel, M.M.; Steele, A.D.; Gentsch, J.R.; Payne, D.C.; Cortese, M.M.; Nakagomi, O.; Cunliffe, N.A.; Jiang, B.; Neuzil, K.M.; et al. Global impact of rotavirus vaccines. Expert Rev. Vaccines 2010, 9, 395–407. [Google Scholar]
- Serazin, A.C.; Shackelton, L.A.; Wilson, C.; Bhan, M.K. Improving the performance of enteric vaccines in the developing world. Nat. Immunol. 2010, 11, 769–773. [Google Scholar]
- Madhi, S.A.; Cunliffe, N.A.; Steele, D.; Witte, D.; Kirsten, M.; Louw, C.; Ngwira, B.; Victor, J.C.; Gillard, P.H.; Cheuvart, B.B.; Han, H.H.; Neuzil, K.M. Effect of human rotavirus vaccine on severe diarrhea in african infants. N. Engl. J. Med. 2010, 362, 289–298. [Google Scholar]
- Theil, K. Group A Rotaviruses. In Viral Diarrheas of Man and Animals; Saif, L., Theil, K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 35–72. [Google Scholar]
- Park, S.H.; Saif, L.J.; Jeong, C.; Lim, G.K.; Park, S.I.; Kim, H.H.; Park, S.J.; Kim, Y.J.; Jeong, J.H.; Kang, M.I.; Cho, K.O. Molecular characterization of novel G5 bovine rotavirus strains. J. Clin. Microbiol. 2006, 44, 4101–4112. [Google Scholar]
- Martella, V.; Banyai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar]
- El-Attar, L.; Oliver, S.L.; Mackie, A.; Charpilienne, A.; Poncet, D.; Cohen, J.; Bridger, J.C. Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease. Vaccine 2009, 27, 3201–3208. [Google Scholar]
- Patel, N.C.; Hertel, P.M.; Estes, M.K.; de la Morena, M.; Petru, A.M.; Noroski, L.M.; Revell, P.A.; Hanson, I.C.; Paul, M.E.; Rosenblatt, H.M.; Abramson, S.L. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N. Engl. J. Med. 2010, 362, 314–319. [Google Scholar]
- Wang, Y.; Azevedo, M.; Saif, L.J.; Gentsch, J.R.; Glass, R.I.; Jiang, B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010, 28, 5432–5436. [Google Scholar]
- van Pinxteren, L.A.; Bruce, M.G.; Campbell, I.; Wood, A.; Clarke, C.J.; Bellman, A.; Morein, B.; Snodgrass, D.R. Effect of oral rotavirus/iscom vaccines on immune responses in gnotobiotic lambs. Vet. Immunol. Immunopathol. 1999, 71, 53–67. [Google Scholar]
- Chang, J.T.; Li, X.; Liu, H.J.; Yu, L. Ovine rotavirus strain LLR-85-based bovine rotavirus candidate vaccines: construction, characterization and immunogenicity evaluation. Vet. Microbiol. 2010, 146, 35–43. [Google Scholar]
- O’Neal, C.M.; Crawford, S.E.; Estes, M.K.; Conner, M.E. Rotavirus virus-like particles administered mucosally induce protective immunity. J. Virol. 1997, 71, 8707–8717. [Google Scholar]
- Yoo, D.; Lee, J.; Harland, R.; Gibbons, E.; Elazhary, Y.; Babiuk, L.A. Maternal immunization of pregnant cattle with recombinant VP8* protein of bovine rotavirus elicits neutralizing antibodies to multiple serotypes. Colostral neutralizing antibody by rotavirus VP8*. Adv. Exp. Med. Biol. 1997, 412, 405–411. [Google Scholar] [PubMed]
- Barrandeguy, M.; Parreno, V.; Lagos Marmol, M.; Pont Lezica, F.; Rivas, C.; Valle, C.; Fernandez, F. Prevention of rotavirus diarrhoea in foals by parenteral vaccination of the mares: field trial. Dev. Biol. Stand. 1998, 92, 253–257. [Google Scholar]
- Saif, L.J. Enteric viral infections of pigs and strategies for induction of mucosal immunity. Adv. Vet. Med. 1999, 41, 429–446. [Google Scholar]
- Kapikian, A.Z.; Simonsen, L.; Vesikari, T.; Hoshino, Y.; Morens, D.M.; Chanock, R.M.; La Montagne, J.R.; Murphy, B.R. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J. Infect. Dis. 2005, 192 (Suppl. 1), S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar]
- Ruiz-Palacios, G.M.; Perez-Schael, I.; Velazquez, F.R.; Abate, H.; Breuer, T.; Clemens, S.C.; Cheuvart, B.; Espinoza, F.; Gillard, P.; Innis, B.L.; et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 2006, 354, 11–22. [Google Scholar]
- Vesikari, T.; Karvonen, A.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Meurice, F.; Han, H.H.; Damaso, S.; Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007, 370, 1757–1763. [Google Scholar]
- Block, S.L.; Vesikari, T.; Goveia, M.G.; Rivers, S.B.; Adeyi, B.A.; Dallas, M.J.; Bauder, J.; Boslego, J.W.; Heaton, P.M. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007, 119, 11–18. [Google Scholar] [PubMed]
- Rolsma, M.D.; Gelberg, H.B.; Kuhlenschmidt, M.S. Assay for evaluation of rotavirus-cell interactions: Identification of an enterocyteganglioside fraction that mediates group a porcine rotavirus recognition. J. Virol. 1994, 68, 258–268. [Google Scholar]
- Kuhlenschmidt, M.S.; Rolsma, M.D.; Kuhlenschmidt, T.B.; Gelberg, H.B. Characterization of a porcine enterocyte receptor for group A rotavirus. Adv. Exp. Med. Biol. 1997, 412, 135–143. [Google Scholar]
- Rolsma, M.D.; Kuhlenschmidt, T.B.; Gelberg, H.B.; Kuhlenschmidt, M.S. Structure and function of a ganglioside receptor for porcine rotavirus. J. Virol. 1998, 72, 9079–9091. [Google Scholar]
- Zijlstra, R.T.; Donovan, S.M.; Odle, J.; Gelberg, H.B.; Petschow, B.W.; Gaskins, H.R. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J. Nutr. 1997, 127, 1118–1127. [Google Scholar]
- Pohlentz, G.; Egge, H. Neoglycolipids of 1-Deoxy-1-phosphatidylethanolaminolactitol type: Synthesis, structure analysis, and use as probes for characterization of glycosyltransferases. Methods Enzymol. 1994, 242, 127–145. [Google Scholar]
- Powell, L.D.; Hart, G.W. Quantitation of picomole levels of N-acetyl- and N-glycoylneuraminic acids by a HPLC-adaptation of the thiobarbituric acid assay. Anal.Biochem. 1986, 157, 179–185. [Google Scholar] [PubMed]
- Chen, P.S.; Toribara, T.Y.; Warner, H. Micro phosphate method. Anal.Chem. 1956, 28, 1756. [Google Scholar]
- Schmidt, J.; Kuhlenschmidt, M.S. Microbial adhesion of Cryptosporidium parvum: Identification of a colostrum-derived inhibitory lipid. Mol. Biochem. Parasitol. 2008, 162, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Laferriere, C.A.; Roy, R. Isolation, modification and conjugation of sialyl alpha (2-3)-lactose. Methods Enzymol. 1994, 242, 102–108. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal.Biochem. 1985, 150, 76–85. [Google Scholar]
- Pohlentz, G.; Schlemm, S.; Klima, B.; Egge, H. Fast atom bombardment mass spectrometry of N-acetylated neoglycolipids of the 1-deoxy-1phosphatidylethanolamino-lactitol type. Chem. Phys. Lipids 1994, 70, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ramig, R.F. Rescue of infectivity by sequential in vitro transcapsidation of rotavirus core particles with inner capsid and outer capsid proteins. Virology 1993, 194, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kuhlenschmidt, T.B.; Hanafin, W.P.; Gelberg, H.B.; Kuhlenschmidt, M.S. Sialic acid dependence and independence of group A rotaviruses. Adv. Exp. Med. Biol. 1999, 473, 309–317. [Google Scholar]
- Karlsson, K.-A.; Stromberg, N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987, 138, 220–232. [Google Scholar]
- Thulin, J.D.; Kuhlenschmidt, M.S.; Gelberg, H.B. Development, characterization, and utilization of an intestinal xenograft model for infectious disease research. Lab. Invest. 1991, 65, 719–730. [Google Scholar] [PubMed]
- Pohlentz, G.; Schlemm, S.; Egge, H. 1-Deoxy-1-phosphatidylethanolamino-lactitol-type neoglycolipids serve as acceptors for sialyltransferases from rat live golgi vesicles. Eur. J. Biochem. 1992, 203, 387–392. [Google Scholar]
- Delorme, C.; Brussow, H.; Sidoti, J.; Roche, N.; Karlsson, K.-A.; Neeser, J.-R.; Teneberg, S. Glycosphingolipid binding specificities of rotavirus: Identification of a sialic acid-binding epitope. J. Virol. 2001, 75, 2276–2287. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bergner, D.W.; Kuhlenschmidt, T.B.; Hanafin, W.P.; Firkins, L.D.; Kuhlenschmidt, M.S. Inhibition of Rotavirus Infectivity by a Neoglycolipid Receptor Mimetic. Nutrients 2011, 3, 228-244. https://0-doi-org.brum.beds.ac.uk/10.3390/nu3020228
Bergner DW, Kuhlenschmidt TB, Hanafin WP, Firkins LD, Kuhlenschmidt MS. Inhibition of Rotavirus Infectivity by a Neoglycolipid Receptor Mimetic. Nutrients. 2011; 3(2):228-244. https://0-doi-org.brum.beds.ac.uk/10.3390/nu3020228
Chicago/Turabian StyleBergner, Daniel W., Theresa B. Kuhlenschmidt, William P. Hanafin, Lawrence D. Firkins, and Mark S. Kuhlenschmidt. 2011. "Inhibition of Rotavirus Infectivity by a Neoglycolipid Receptor Mimetic" Nutrients 3, no. 2: 228-244. https://0-doi-org.brum.beds.ac.uk/10.3390/nu3020228