Carbohydrate Electrolyte Solutions Enhance Endurance Capacity in Active Females
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Preliminary Measurements
2.3. Experimental Procedures
2.4. Statistical Analysis
3. Results
3.1. Exercise Time to Exhaustion
3.2. Expired Air Analysis
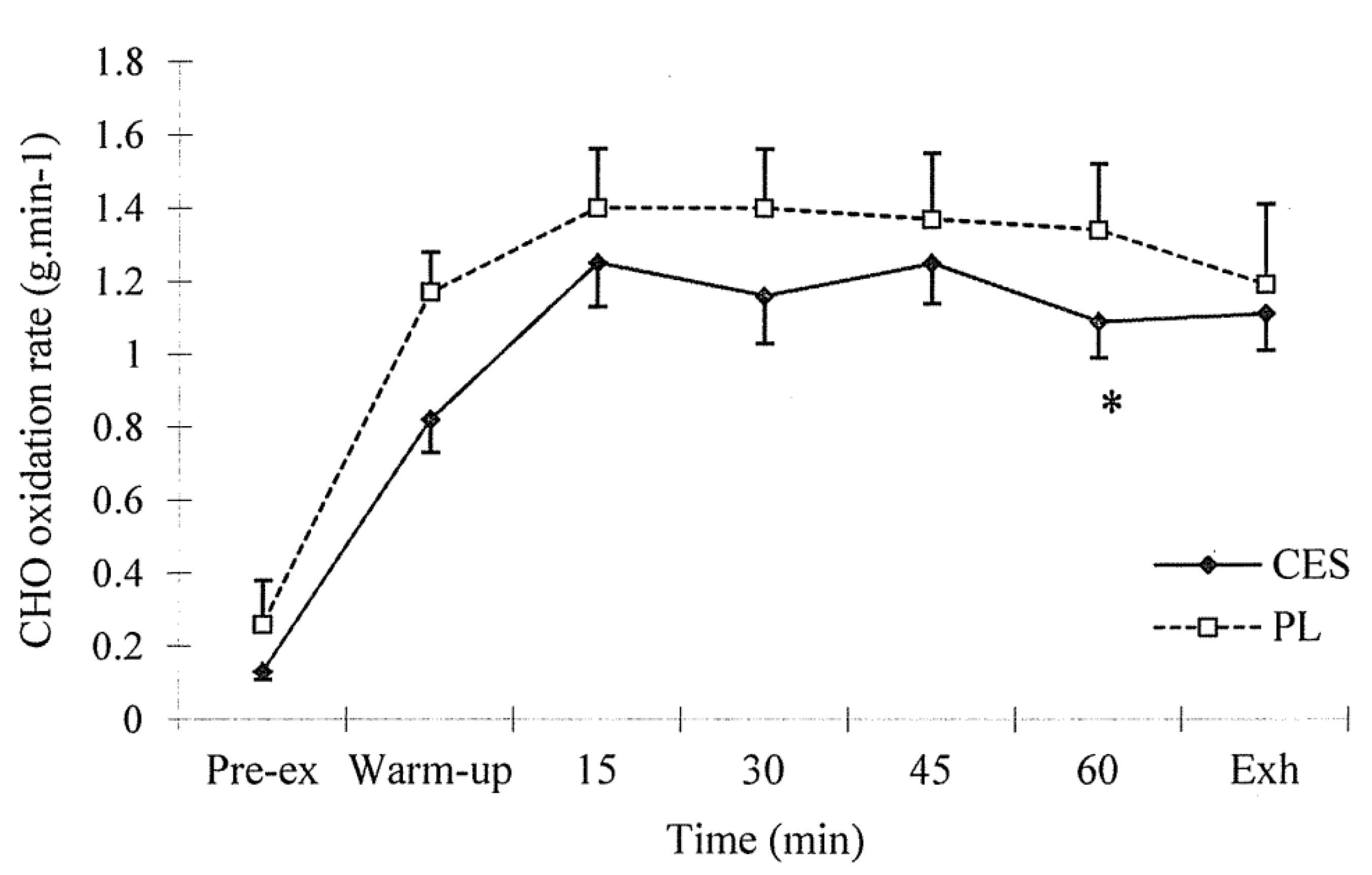
3.3. Blood Sampling Analysis
3.4. Heart Rate, Ratings of Perceived Exertion, Thirst and Abdominal Discomfort

| Trial | Run Time (min) | Significance Level | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-ex | Warm-up | 15th | 30th | 45th | 60th | EXH | |||
| Heart Rate (beats·min−1) | CES | 63.9 ± 2.1 | 115.4 ± 9.5 | 148.4 ± 3.9 | 150.0 ± 3.5 | 150.9 ± 3.4 | 151.4 ± 3.5 | 151.3 ± 3.2 | F = 2.047 |
| PL | 64.0 ± 3.1 | 135.9 ± 9.4 | 148.4 ± 2.9 | 150.0 ± 3.3 | 150.5 ± 3.7 | 152.4 ± 3.5 | 152.4 ± 4.4 | p = 0.08 | |
| RPE (Borg Scale) | CES | 7.1 ± 0.4 | 7.5 ± 0.4 | 10.3 ± 0.6 | 11.3 ± 0.7 | 13.8 ± 0.6 | 14.3 ± 1.0 | 18.5 ± 0.6 | F = 1.767 |
| PL | 8.0 ± 0.6 | 9.4 ± 0.5 | 10.9 ± 0.7 | 12.4 ± 0.6 | 13.3 ± 0.7 | 15.3 ± 0.8 | 18.9 ± 0.4 | p = 0.129 | |
| PTS | CES | 2.1 ± 0.5 | 3.5 ± 0.7 | 3.1 ± 0.6 | 3.3 ± 0.8 | 3.9 ± 0.8 | 4.3 ± 0.9 | F = 1.161 | |
| PL | 3.0 ± 0.8 | 4.1 ± 0.8 | 3.4 ± 0.9 | 3.4 ± 0.9 | 4.3 ± 0.9 | 3.9 ± 1.1 | p = 0.347 | ||
| PAS | CES | 0.3 ± 0.3 | 0.6 ± 0.4 | 1.6 ± 0.6 | 2.4 ± 0.5 | 3.3 ± 0.7 | 3.5 ± 0.7 | 4.0 ± 0.9 | F = 0.046 |
| PL | 0.4 ± 0.3 | 0.9 ± 0.5 | 1.5 ± 0.6 | 2.4 ± 0.7 | 3.1 ± 0.8 | 3.4 ± 0.8 | 4.0 ± 0.3 | p = 0.999 | |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coyle, E.F. Substrate utilization during exercise in active people. Am. J. Clin. Nutr. 1995, 61, S968–S979. [Google Scholar]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Tsintzas, K.; Williams, C.; Wilson, W.; Burrin, J. Influence of carbohydrate supplementation early in exercise on endurance running capacity. Med. Sci. Sports Exerc. 1996, 28, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Temesi, J.; Johnson, N.A.; Raymond, J.; Burdon, C.A.; O’Connor, H.T. Carbohydrate ingestion during endurance exercise improves performance in adults. J. Nutr. 2011, 141, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Smith, J.E.W.; Passe, D.H.; Péronnet, F. Carbohydrate administration and exercise performance: What are the potential mechanisms involved? Sports Med. 2010, 40, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.; van Loon, L.C. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.L.; Sedlock, D.A.; Flynn, M.G.; Navalta, J.W.; Ji, H. Carbohydrate loading and supplementation in endurance-trained women runners. J. Appl. Physiol. 2003, 95, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.P.; Zacher, C.M.; Mittleman, K.D. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J. Appl. Physiol. 2000, 88, 690–697. [Google Scholar] [PubMed]
- Campbell, S.E.; Angus, D.J.; Febbraio, M.A. Glucose kinetics and exercise performance during phases of the menstrual cycle. Am. J. Physiol. Endocrin. Metab. 2001, 281, E817–E825. [Google Scholar]
- Tarnopolsky, L.J.; MacDougall, J.D.; Atkinson, S.A.; Tarnopolsky, M.A.; Sutton, J.R. Gender differences in substrate for endurance exercise. J. Appl. Physiol. 1990, 68, 302–308. [Google Scholar] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Zaltas, E.; Jeukendrup, A.E. Oxidation of solid versus liquid CHO sources during exercise. Med. Sci. Sports Exerc. 2010, 42, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Zaltas, E.; Jeukendrup, A.E. CHO oxidation from a CHO gel compared with a drink during exercise. Med. Sci. Sports Exerc. 2010, 42, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Impact of mild dehydration on wellness and on exercise performance. Eur. J. Clin. Nutr. 2003, 57, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Coyle, E.F. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J. Appl. Physiol. 1992, 73, 1340–1350. [Google Scholar] [PubMed]
- Nose, H.; Mack, G.W.; Shi, X.R.; Nadel, E.R. Role of osmolality and plasma volume during rehydration in humans. J. Appl. Physiol. 1988, 65, 325–331. [Google Scholar] [PubMed]
- Wong, S.H.S.; Williams, C.; Adams, N. Effects of ingesting a large volume of carbohydrate-electrolyte solution on rehydration during recovery and subsequent exercise capacity. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 375–393. [Google Scholar] [PubMed]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [PubMed]
- Wong, S.H.S.; Williams, C.; Simpson, M.; Ogaki, T. Influence of fluid intake pattern on short-term recovery from prolonged, submaximal running and subsequent exercise capacity. J. Sports Sci. 1998, 16, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Currell, K.; Jeukendrup, A.E. Validity, reliability and sensitivity of measures of sporting performance. Sports Med. 2008, 38, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Gore, C.J.; Scroop, G.C.; Marker, J.D.; Catcheside, P.G. Plasma volume, osmolarity, total protein and electrolytes during treadmill running and cycle ergometer exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Horton, T.J.; Grunwald, G.K.; Lavely, J.; Donahoo, W.T. Glucose kinetics differ between women and men, during and after exercise. J. Appl. Physiol. 2006, 100, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.; Peronnet, F.; Massicotte, D.; Lavoie, C. Carbohydrate supplementation and sex differences in fuel selection during exercise. Med. Sci. Sport. Exerc. 2010, 42, 1314–1323. [Google Scholar] [CrossRef]
- Wallis, G.A.; Dawson, R.; Achten, J.; Webber, J.; Jeukendrup, A.E. Metabolic response to carbohydrate ingestion during exercise in males and females. Am. J. Physiol. Endocrin. Metab. 2006, 290, E708–E715. [Google Scholar] [CrossRef]
- IOC. IOC consensus statement on sports nutrition 2010. J. Sports Sci. 2011, 29, S3–S4. [Google Scholar]
- Candas, V.; Libert, J.P.; Brandenberger, G.; Sagot, J.C.; Amoros, C.; Kahn, J.M. Hydration during exercise: Effects on thermal and cardiovascular adjustments. Eur. J. Appl. Physiol. 1986, 55, 113–122. [Google Scholar] [CrossRef]
- Shirreffs, S.M.; Aragon-Vargas, L.F.; Keil, M.; Love, T.D.; Phillips, S. Rehydration after exercise in the heat: A comparison of 4 commonly used drinks. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 244–258. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.-H.; Wong, S.H.-S.; Chen, S.-H.; Poon, T.-C. Carbohydrate Electrolyte Solutions Enhance Endurance Capacity in Active Females. Nutrients 2015, 7, 3739-3750. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7053739
Sun F-H, Wong SH-S, Chen S-H, Poon T-C. Carbohydrate Electrolyte Solutions Enhance Endurance Capacity in Active Females. Nutrients. 2015; 7(5):3739-3750. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7053739
Chicago/Turabian StyleSun, Feng-Hua, Stephen Heung-Sang Wong, Shi-Hui Chen, and Tsz-Chun Poon. 2015. "Carbohydrate Electrolyte Solutions Enhance Endurance Capacity in Active Females" Nutrients 7, no. 5: 3739-3750. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7053739





