The Tanita SC-240 to Assess Body Composition in Pre-School Children: An Evaluation against the Three Component Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Measures
2.2.1. Anthropometry
2.2.2. Bioelectrical Impedance
2.2.3. Three Component Model
2.3. Statistical Analysis
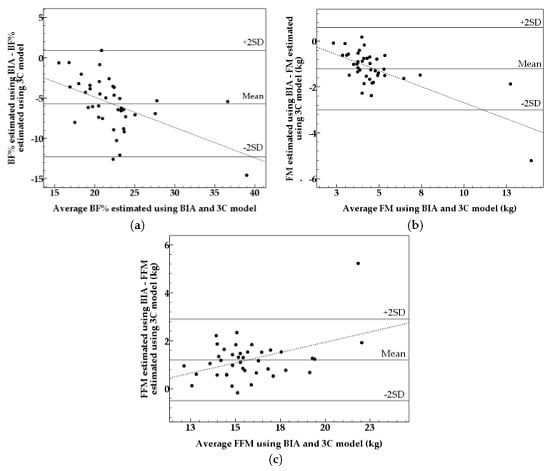
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BF% | Body Fat Percentage |
| FM | Fat Mass |
| FFM | Fat Free Mass |
| TBW | Total Body Water |
| ADP | Air Displacement Plethysmography |
| BIA | Bioelectrical Impedance |
| DXA | Dual Energy X-ray Absorptiometry |
| 2C Model | Two Component Model |
| 3C Model | Three Component Model |
| 4C Model | Four Component Model |
| SD | Standard Deviation |
References
- World Health Organization. Facts and Figures on Childhood Obesity. Available online: http://www.who.int/end-childhood-obesity/facts/en/ (accessed on 14 December 2015).
- World Health Organization. Obesity. Available online: http://www.who.int/topics/obesity/en/ (accessed on 15 December 2015).
- Ellis, K.J.; Abrams, S.A.; Wong, W.W. Monitoring childhood obesity: Assessment of the weight/height index. Am. J. Epidemiol. 1999, 150, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Forsum, E.; Carlsson, E.F.; Henriksson, H.; Henriksson, P.; Lof, M. Total body fat content versus BMI in 4-year-old healthy Swedish children. J. Obes. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2006, 91, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Barreira, T.V.; Staiano, A.E.; Katzmarzyk, P.T. Validity assessment of a portable bioimpedance scale to estimate body fat percentage in white and African-American children and adolescents. Pediatr. Obes. 2013, 8, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, B.S.; Stockton, M.B.; Lanctot, J.Q.; Relyea, G.; Klesges, R.C.; Slawson, D.L.; Schilling, L.P. Measurement of body composition in 8–10-year-old African-American girls: A comparison of dual-energy X-ray absorptiometry and foot-to-foot bioimpedance methods. Int. J. Pediatr. Obes. 2009, 4, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hosking, J.; Metcalf, B.S.; Jeffery, A.N.; Voss, L.D.; Wilkin, T.J. Validation of foot-to-foot bioelectrical impedance analysis with dual-energy X-ray absorptiometry in the assessment of body composition in young children: The earlybird cohort. Br. J. Nutr. 2006, 96, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Delisle, C.; Sandin, S.; Forsum, E.; Henriksson, H.; Trolle-Lagerros, Y.; Larsson, C.; Maddison, R.; Ortega, F.B.; Ruiz, J.R.; Silfvernagel, K.; et al. A web- and mobile phone-based intervention to prevent obesity in 4-year-old (ministop): A population-based randomized controlled trial. BMC Public Health 2015, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, C.D.; Forsum, E.; Henriksson, H.; Trolle-Lagerros, Y.; Larsson, C.; Maddison, R.; Timpka, T.; Lof, M. A mobile phone based method to assess energy and food intake in young children: A validation study against the doubly labelled water method and 24 h dietary recalls. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- Cadenas-Sanchez, C.; Nystrom, C.D.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Mora-Gonzalez, J.; Risinger, A.S.; Ruiz, J.R.; Ortega, F.B.; Lof, M. Prevalence of overweight/obesity and fitness level in preschool children from the north compared with the south of Europe: An exploration with two countries. Pediatr. Obes. 2015. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Wikland, K.A.; Luo, Z.C.; Niklasson, A.; Karlberg, J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 2002, 91, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Fuller, N.J.; Jebb, S.A.; Laskey, M.A.; Coward, W.A.; Elia, M. Four-component model for the assessment of body composition in humans: Comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin. Sci. 1992, 82, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.H.; Nyström, C.D.; Henriksson, P.; Pomeroy, J.; Ruiz, J.R.; Ortega, F.B.; Cadenas-Sánchez, C.; Löf, M. Physical activity intensity, sedentary behavior, body composition and physical fitness in 4-year-old children: Results from the MINISTOP trial. Int. J. Obes. 2016. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques; IAEA Human Health Series No. 3; IAEA International Atomic Energy Agency: Vienna, Austria, 2009. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Kabiri, L.S.; Hernandez, D.C.; Mitchell, K. Reliability, validity, and diagnostic value of a pediatric bioelectrical impedance analysis scale. Child. Obes. 2015, 11, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Tompuri, T.T.; Lakka, T.A.; Hakulinen, M.; Lindi, V.; Laaksonen, D.E.; Kilpelainen, T.O.; Jaaskelainen, J.; Lakka, H.M.; Laitinen, T. Assessment of body composition by dual-energy X-ray absorptiometry, bioimpedance analysis and anthropometrics in children: The physical activity and nutrition in children study. Clin. Physiol. Funct. Imaging 2015, 35, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.A.; Salazar, G.D.; de Romana, D.V.L.; Kain, J.A.; Corvalan, C.L.; Uauy, R.E. Evaluation of simple body composition methods: Assessment of validity in prepubertal Chilean children. Eur. J. Clin. Nutr. 2015, 69, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; DeLany, J.P.; Volaufova, J.; Harsha, D.W.; Champagne, C. Prediction of body fat in 12-year-old African American and white children: Evaluation of methods. Am. J. Clin. Nutr. 2002, 76, 980–990. [Google Scholar] [PubMed]
- Bammann, K.; Huybrechts, I.; Vicente-Rodriguez, G.; Easton, C.; de Vriendt, T.; Marild, S.; Mesana, M.I.; Peeters, M.W.; Reilly, J.J.; Sioen, I.; et al. Validation of anthropometry and foot-to-foot bioelectrical resistance against a three-component model to assess total body fat in children: The IDEFICS study. Int. J. Obes. (Lond.) 2013, 37, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Luque, V.; Closa-Monasterolo, R.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Ferre, N.; Gispert-Llaurado, M.; Escribano, J. Bioimpedance in 7-year-old children: Validation by dual X-ray absorptiometry—Part 1: Assessment of whole body composition. Ann. Nutr. Metab. 2014, 64, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Earthman, C.P.; Pichard, C.; Coss-Bu, J.A. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2015, 69, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Fuller, N.J.; Dewit, O.; Fewtrell, M.S.; Elia, M.; Cole, T.J. Four-component model of body composition in children: Density and hydration of fat-free mass and comparison with simpler models. Am. J. Clin. Nutr. 1999, 69, 904–912. [Google Scholar] [PubMed]


| Variable | BIA | 3C Model | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Age (years) | 5.5 ± 0.2 | 5.2–5.7 | - | - |
| Weight (kg) | 20.5 ± 4.2 | 14.9–35.8 | - | - |
| Weight for age z-score 1 | −0.05 ± 1.55 | −2.22–5.41 | - | - |
| Height (cm) | 114.0 ± 4.0 | 105.0–125.5 | - | - |
| Height for age z-score 1 | 0.00 ± 0.90 | −1.92–2.26 | - | - |
| BMI (kg/m2) 2 | 15.6 ± 2.3 | 13.3–25.6 | - | - |
| Body fat percentage | 19.4 ± 3.9 * | 13.5–33.9 | 25.1 ± 5.5 | 15.8–46.3 |
| Fat mass (kg) | 4.1 ± 1.9 * | 2.3–11.8 | 5.3 ± 2.5 | 2.4–16.6 |
| Fat free mass (kg) | 16.4 ± 2.4 * | 12.6–24.5 | 15.2 ± 2.0 | 11.7–21.1 |
| FMI (kg/m2) | 3.1 ± 1.2 * | 1.8–8.1 | 4.0 ± 1.7 | 2.1–11.8 |
| FFMI (kg/m2) | 12.5 ± 1.1 * | 10.8–17.5 | 11.6 ± 0.8 | 10.0–13.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delisle Nyström, C.; Henriksson, P.; Alexandrou, C.; Löf, M. The Tanita SC-240 to Assess Body Composition in Pre-School Children: An Evaluation against the Three Component Model. Nutrients 2016, 8, 371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu8060371
Delisle Nyström C, Henriksson P, Alexandrou C, Löf M. The Tanita SC-240 to Assess Body Composition in Pre-School Children: An Evaluation against the Three Component Model. Nutrients. 2016; 8(6):371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu8060371
Chicago/Turabian StyleDelisle Nyström, Christine, Pontus Henriksson, Christina Alexandrou, and Marie Löf. 2016. "The Tanita SC-240 to Assess Body Composition in Pre-School Children: An Evaluation against the Three Component Model" Nutrients 8, no. 6: 371. https://0-doi-org.brum.beds.ac.uk/10.3390/nu8060371