Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions
Abstract
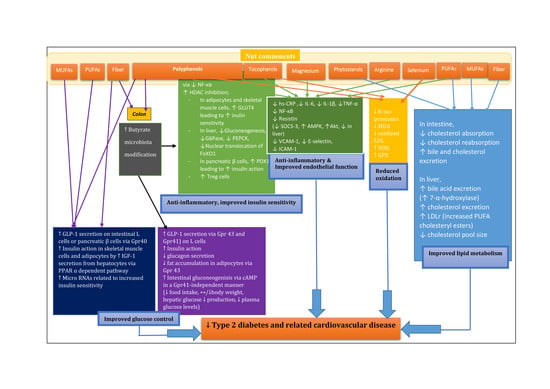
:1. Introduction
2. Nut Composition
3. Glycemic Control
3.1. Acute Studies
3.2. Possible Nutrients Involved in Glucose-Lowering Effects in Nuts
3.3. Modification in Micro RNAs Related to Insulin Sensitivity
4. Body Weight Control, Appetite, Energy Balance and Lipid Bioaccessibility
4.1. Body Weight Control
4.2. Appetite
4.3. Energy Balance
4.4. Bioaccessibility of Nutrients
5. Gut Microbiota Modification
5.1. Urolithin (Microflora Metabolite)
5.2. Butyrate (Microflora Metabolite)
6. Changes in Blood Lipids and Lipoproteins
6.1. Unsaturated Fat
6.2. The Lysine to Arginine Ratio
6.3. Phytosterols
6.4. Fiber
7. Antioxidant Activity and Decreased Oxidative Stress
8. Anti-Inflammatory Actions
9. Endothelial Function
10. Blood Pressure
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.; Anderson, R.; Buse, J.; Chin, M.; Eddy, D.; Fradkin, J.; Ganiats, T.; Ginsberg, H.; Kahn, R.; Nwankwo, R. The prevention or delay of type 2 diabetes. Diabetes Care 2003, 26, S62. [Google Scholar] [PubMed]
- Nanditha, A.; Ma, R.C.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in asia and the pacific: Implications for the global epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Dagenais, G.R.; Mohan, V.; Diaz, R.; Probstfield, J.; Freeman, R.; Shaw, J.; Lanas, F.; Avezum, A.; Budaj, A.; et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: The epidream cohort study. Eur. J. Prev. Cardiol. 2012, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kirpichnikov, D.; Sowers, J.R. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol. Metab. 2001, 12, 225–230. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Pfeiffer, A.F. Foods for the prevention of diabetes: How do they work? Diabetes Metab. Res. Rev. 2012, 28, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegl, K.; Haddad, E.; Oda, K.; Fraser, G.E.; Sabate, J. Tree nuts are inversely associated with metabolic syndrome and obesity: The adventist health study-2. PLoS ONE 2014, 9, e85133. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; de Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A systematic review and meta-analysis of nut consumption and incident risk of cvd and all-cause mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.; Neuringer, M.; SanGiovanni, J.P. Macular xanthophylls, lipoprotein-related genes, and age-related macular degeneration. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 336S–346S. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Agricultural Research Service USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 10 March 2017).
- Phenol Explorer Database on Polyphenol Content in Foods. Available online: http://www.phenol-explorer.eu (accessed on 24 May 2017).
- Yang, J. Brazil nuts and associated health benefits: A review. Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Viguiliouk, E.; Kendall, C.W.; Blanco Mejia, S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- Zibaeenezhad, M.; Aghasadeghi, K.; Hakimi, H.; Yarmohammadi, H.; Nikaein, F. The effect of walnut oil consumption on blood sugar in patients with diabetes mellitus type 2. Int. J. Endocrinol. Metab. 2016, 14, e34889. [Google Scholar] [PubMed]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: A randomized trial. Metabolism 2015, 64, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized crossover trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.W.; Balasubramanyam, A.; Kimball, K.T.; Aherns, A.K.; Fordis, C.M., Jr.; Ballantyne, C.M. Long-term, randomized clinical trial of two diets in the metabolic syndrome and type 2 diabetes. Diabetes Care 2003, 26, 2481–2482. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.A.; Sabate, J.M.; Ikle, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs. complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Josse, A.R.; Esfahani, A.; Jenkins, D.J. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur. J. Clin. Nutr. 2011, 65, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; West, S.G.; Augustin, L.S.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.A.; Campbell, J.; Chiavaroli, L.; Jenkins, A.L.; et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.; Ribeiro, D.N.; Costa, N.M.; Bressan, J.; Alfenas, R.C.; Mattes, R.D. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: A randomised cross-over clinical trial. Br. J. Nutr. 2013, 109, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Buller, A.J. Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J. Am. Diet. Assoc. 2005, 105, 1939–1942. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [PubMed]
- Josse, A.R.; Kendall, C.W.; Augustin, L.S.; Ellis, P.R.; Jenkins, D.J. Almonds and postprandial glycemia—A dose-response study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Crouch, M.A.; Slater, R.T., III. Almond “appetizer” effect on glucose tolerance test (GTT) results. J. Am. Board Fam. Med. 2016, 29, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Esfahani, A.; Josse, A.R.; Augustin, L.S.; Vidgen, E.; Jenkins, D.J. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Most, M.M.; Lefevre, M.; Greenway, F.L.; Rood, J.C. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 1000–1006. [Google Scholar] [PubMed]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Wendland, E.; Farmer, A.; Glasziou, P.; Neil, A. Effect of α linolenic acid on cardiovascular risk markers: A systematic review. Heart 2006, 92, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Yatsuya, H.; Toyoshima, H.; Sasaki, S.; Li, Y.; Otsuka, R.; Wada, K.; Hotta, Y.; Mitsuhashi, H.; Matsushita, K.; et al. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged japanese. Prev. Med. 2010, 50, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Swaminath, G.; Cao, Q.; Yang, L.; Guo, Q.; Salomonis, H.; Lu, J.; Houze, J.B.; Dransfield, P.J.; Wang, Y.; et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol. Cell. Endocrinol. 2013, 369, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Feng, D.D.; Wang, D.F.; Sun, J.; Li, S.B.; Chen, C. Long-term in vitro treatment of INS-1 rat pancreatic beta-cells by unsaturated free fatty acids protects cells against gluco- and lipotoxicities via activation of GPR40 receptors. Clin. Exp. Pharmacol. Physiol. 2012, 39, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.L.; Shu, G.; Zhang, Z.Q.; Wang, S.B.; Zhu, X.T.; Gao, P.; Xi, Q.Y.; Zhang, Y.L.; Jiang, Q.Y. Roles of alpha-linolenic acid on IGF-I secretion and GH/IGF system gene expression in porcine primary hepatocytes. Mo. Biol. Rep. 2012, 39, 10987–10996. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J. Clin. Investig. 2004, 113, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramirez-Chavez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common micrornas. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Giardina, S.; Salas-Salvado, J.; Arcelin, P.; Bullo, M. Chronic pistachio intake modulates circulating micrornas related to glucose metabolism and insulin resistance in prediabetic subjects. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of micrornas in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Alper, C.M.; Mattes, R.D. Effects of chronic peanut consumption on energy balance and hedonics. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002, 26, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Bennett, H.W.; Jaceldo, K.B.; Sabate, J. Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months. J. Am. Coll. Nutr. 2002, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.; Mattes, R. Effect of chronic consumption of almonds on body weight in healthy humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Almario, R.U.; Vonghavaravat, V.; Wong, R.; Kasim-Karakas, S.E. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am. J. Clin. Nutr. 2001, 74, 72–79. [Google Scholar] [PubMed]
- Abbey, M.; Noakes, M.; Belling, G.B.; Nestel, P.J. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am. J. Clin. Nutr. 1994, 59, 995–999. [Google Scholar] [PubMed]
- Coelho, S.B.; de Sales, R.L.; Iyer, S.S.; Bressan, J.; Costa, N.M.; Lokko, P.; Mattes, R. Effects of peanut oil load on energy expenditure, body composition, lipid profile, and appetite in lean and overweight adults. Nutrition 2006, 22, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ 1998, 317, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Gaziano, J.M.; Willett, W.C.; Manson, J.E. Nut consumption and decreased risk of sudden cardiac death in the physicians’ health study. Arch. Intern. Med. 2002, 162, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Sabate, J.; Beeson, W.L.; Strahan, T.M. A possible protective effect of nut consumption on risk of coronary heart disease. The adventist health study. Arch. Intern. Med. 1992, 152, 1416–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellsworth, J.L.; Kushi, L.H.; Folsom, A.R. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: The iowa women’s health study. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 372–377. [Google Scholar] [PubMed]
- Bes-Rastrollo, M.; Wedick, N.M.; Martinez-Gonzalez, M.A.; Li, T.Y.; Sampson, L.; Hu, F.B. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am. J. Clin. Nutr. 2009, 89, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Bes-Rastrollo, M.; Sabate, J.; Gomez-Gracia, E.; Alonso, A.; Martinez, J.A.; Martinez-Gonzalez, M.A. Nut consumption and weight gain in a Mediterranean cohort: The sun study. Obesity 2007, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Bullo, M.; Ros, E.; Basora, J.; Salas-Salvado, J. Cross-sectional association of nut intake with adiposity in a Mediterranean population. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; et al. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A.; Jenkins, D.A.; Bosello, O.; Gates, J.E.; Cragen, L.N.; Bruce, B. Nuts and plasma lipids: An almond-based diet lowers LDL-C while preserving HDL-C. J. Am. Coll. Nutr. 1998, 17, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A.; Jenkins, D.J.; Cragen, L.N.; Gates, J.E.; Bosello, O.; Berra, K.; Rudd, C.; Stevenson, M.; Superko, R. Effect of a diet high in monounsaturated fat from almonds on plasma cholesterol and lipoproteins. J. Am. Coll. Nutr. 1992, 11, 126–130. [Google Scholar] [PubMed]
- Hyson, D.A.; Schneeman, B.O.; Davis, P.A. Almonds and almond oil have similar effects on plasma lipids and ldl oxidation in healthy men and women. J. Nutr. 2002, 132, 703–707. [Google Scholar] [PubMed]
- Sabate, J.; Cordero-Macintyre, Z.; Siapco, G.; Torabian, S.; Haddad, E. Does regular walnut consumption lead to weight gain? Br. J. Nutr. 2005, 94, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.; Mann, J.; Skeaff, M.; Frampton, C.; Sutherland, W.; Duncan, A.; Tiszavari, S. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur. J. Clin. Nutr. 1998, 52, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zambon, D.; Sabate, J.; Munoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann. Intern. Med. 2000, 132, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Horton, K.; Reese, D.; Carey, C.; Walker, K.; Capuzzi, D.M. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int. J. Vitam. Nutr. Res. 2002, 72, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.A.; Clayshulte, B.J. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J. Am. Diet. Assoc. 2000, 100, 312–318. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koylu, A.A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Kwaw, I.; Matud, J.; Kurtz, I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. J. Am. Coll. Nutr. 1999, 18, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in chinese subjects with metabolic syndrome. Nutr. J. 2012, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Holligan, S.D.; West, S.G.; Gebauer, S.K.; Kay, C.D.; Kris-Etherton, P.M. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated ldl levels. Br. J. Nutr. 2014, 112, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Curb, J.D.; Wergowske, G.; Dobbs, J.C.; Abbott, R.D.; Huang, B. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch. Intern. Med. 2000, 160, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.; Humphries, J.; Moores, D.; Somerset, S. Effects of a macadamia nut enriched diet on serum lipids and lipoproteins compared to a low fat diet. Food Aust. 1996, 48, 216–222. [Google Scholar]
- Durak, I.; Koksal, I.; Kacmaz, M.; Buyukkocak, S.; Cimen, B.M.; Ozturk, H.S. Hazelnut supplementation enhances plasma antioxidant potential and lowers plasma cholesterol levels. Clin. Chim. Acta 1999, 284, 113–115. [Google Scholar] [CrossRef]
- Razquin, C.; Sanchez-Tainta, A.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Fito, M.; Ros, E.; Estruch, R.; Aros, F.; Gomez-Gracia, E.; et al. Dietary energy density and body weight changes after 3 years in the predimed study. Int. J. Food Sci. Nutr. 2017, 68, 865–872. [Google Scholar] [CrossRef] [PubMed]
- McManus, K.; Antinoro, L.; Sacks, F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Pelkman, C.L.; Fishell, V.K.; Maddox, D.H.; Pearson, T.A.; Mauger, D.T.; Kris-Etherton, P.M. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am. J. Clin. Nutr. 2004, 79, 204–212. [Google Scholar] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.E.J.; Bloom, S.R.; Tan, T. Gut Hormones. In Encyclopedia of Food and Health; Benjamin, C., Paul, M.F., Fidel, T., Eds.; Elsevier Ltd.: Oxford, UK, 2016. [Google Scholar]
- Frost, G.; Brynes, A.E.; Ellis, S.; Milton, J.E.; Nematy, M.; Philippou, E. Nutritional influences on gut hormone release. Curr. Opin. Endocrinol. Diabetes 2006, 13, 42–48. [Google Scholar] [CrossRef]
- Pasman, W.J.; Heimerikx, J.; Rubingh, C.M.; van den Berg, R.; O’Shea, M.; Gambelli, L.; Hendriks, H.F.; Einerhand, A.W.; Scott, C.; Keizer, H.G.; et al. The effect of korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Mantzoros, C.S. Drug insight: The role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef] [PubMed]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Frecka, J.M.; Hollis, J.H.; Mattes, R.D. Effects of appetite, bmi, food form and flavor on mastication: Almonds as a test food. Eur. J. Clin. Nutr. 2008, 62, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Ridgen, J.E.; Phang, P.T.; Birmingham, C.L. Influence of dietary fat polyunsaturated to saturated ratio on energy substrate utilization in obesity. Metabolism 1992, 41, 396–401. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martinez-Gonzalez, M.A.; Castaner, O.; Bullo, M.; Corella, D.; Aros, F.; Gomez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the predimed randomized trial. Can. Med. Assoc. J. 2014, 186, E649–E657. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, E.; Carvajal-Lerida, I.; Perez-Galvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.R.; Kendall, C.W.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [PubMed]
- Mandalari, G.; Grundy, M.M.-L.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Laganà, G.; et al. The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 2014, 112, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; Jenkins, D.; Marchie, A.; Ren, Y.; Ellis, P.; Lapsley, K. Energy Availability from Almonds: Implications for Weight Loss and Cardiovascular Health. A Randomized Controlled Dose-Response Trial. FASEB J. 2003, 17, A339. [Google Scholar]
- Haddad, E.; Sabate, J. Effect of Pecan Consumption on Stool Fat. FASEB J. 2000, 14, A294. [Google Scholar]
- Levine, A.S.; Silvis, S.E. Absorption of whole peanuts, peanut oil, and peanut butter. N. Engl. J. Med. 1980, 303, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Traoret, C.J.; Lokko, P.; Cruz, A.C.; Oliveira, C.G.; Costa, N.M.; Bressan, J.; Alfenas, R.C.; Mattes, R.D. Peanut digestion and energy balance. Int. J. Obes. 2008, 32, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Gibney, M.; Williams, C.M. Postprandial lipoprotein metabolism. Nutr. Res. Rev. 1993, 6, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F. Postprandial lipid metabolism in relation to coronary heart disease. Proc. Nutr. Soc. 1997, 56, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Bassenge, E.; Kim, K.-B.; Kim, Y.-N.; Kim, K.-S.; Lee, H.-J.; Moon, K.-C.; Lee, M.-S.; Park, K.-Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Berry, S.E.; Tydeman, E.A.; Lewis, H.B.; Phalora, R.; Rosborough, J.; Picout, D.R.; Ellis, P.R. Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects. Am. J. Clin. Nutr. 2008, 88, 922–929. [Google Scholar] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Bittner, K.; Rzeppa, S.; Humpf, H.U. Distribution and quantification of flavan-3-ols and procyanidins with low degree of polymerization in nuts, cereals, and legumes. J. Agric. Food Chem. 2013, 61, 9148–9154. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; Gonzalez, C.A.; Buijsse, B.; Guevara, M.; van der Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in european populations. J. Nutr. 2014, 144, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; Gonzalez, C.A.; Buijsse, B.; Guevara, M.; van der Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in european populations: The epic-interact study. Diabetes Care 2013, 36, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and fecal odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Gonzalez-Barrio, R.; Cerda, B.; Lopez-Bote, C.; Rey, A.I.; Tomas-Barberan, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Garrido, I.; Monagas, M.; Gomez-Cordoves, C.; Medina-Remon, A.; Andres-Lacueva, C.; Bartolome, B. Profile of plasma and urine metabolites after the intake of almond [prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. J. Agric. Food Chem. 2009, 57, 10134–10142. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin b2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Llorach, R.; Jauregui, O.; Lopez-Uriarte, P.; Garcia-Aloy, M.; Bullo, M.; Salas-Salvado, J.; Andres-Lacueva, C. Metabolomics unveils urinary changes in subjects with metabolic syndrome following 12-week nut consumption. J. Proteome Res. 2011, 10, 5047–5058. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Urpi-Sarda, M.; Garcia-Villalba, R.; Rabassa, M.; Lopez-Uriarte, P.; Bullo, M.; Jauregui, O.; Tomas-Barberan, F.; Salas-Salvado, J.; Espin, J.C.; et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Uriarte, P.; Nogues, R.; Saez, G.; Bullo, M.; Romeu, M.; Masana, L.; Tormos, C.; Casas-Agustench, P.; Salas-Salvado, J. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clin. Nutr. 2010, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Blaiotta, G.; La Gatta, B.; Di Capua, M.; Di Luccia, A.; Coppola, R.; Aponte, M. Effect of chestnut extract and chestnut fiber on viability of potential probiotic lactobacillus strains under gastrointestinal tract conditions. Food Microbiol. 2013, 36, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding g protein-coupled receptor, GPR41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Stafford, S.; Coope, G.; Heffron, H.; Real, K.; Newman, R.; Davenport, R.; Barnes, M.; Grosse, J.; Cox, H. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes 2015, 64, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. Exenatide and liraglutide: Different approaches to develop GLP-1 receptor agonists (incretin mimetics)—Preclinical and clinical results. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troy, S.; Soty, M.; Ribeiro, L.; Laval, L.; Migrenne, S.; Fioramonti, X.; Pillot, B.; Fauveau, V.; Aubert, R.; Viollet, B.; et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008, 8, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Mithieux, G.; Misery, P.; Magnan, C.; Pillot, B.; Gautier-Stein, A.; Bernard, C.; Rajas, F.; Zitoun, C. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005, 2, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Stein, A.; Zitoun, C.; Lalli, E.; Mithieux, G.; Rajas, F. Transcriptional regulation of the glucose-6-phosphatase gene by cAMP/vasoactive intestinal peptide in the intestine. Role of HNF4alpha, CREM, HNF1alpha, and C/EbPalpha. J. Biol. Chem. 2006, 281, 31268–31278. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin a attenuate nuclear factor kappab activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Joo, M.; Pedchenko, T.; Blackwell, T.S.; Christman, J.W. Regulation of macrophage cyclooxygenase-2 gene expression by modifications of histone H3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L956–L962. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in ifn-gamma-stimulated raw 264.7 murine macrophage cells: Involvement of NF-kappab and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.C.; Vidali, G.; Mann, R.S.; Allfrey, V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978, 253, 3364–3366. [Google Scholar] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485s–2493s. [Google Scholar] [PubMed]
- Wang, X.; Wei, X.; Pang, Q.; Yi, F. Histone deacetylases and their inhibitors: Molecular mechanisms and therapeutic implications in diabetes mellitus. Acta Pharm. Sin. B 2012, 2, 387–395. [Google Scholar] [CrossRef]
- Christensen, D.P.; Dahllof, M.; Lundh, M.; Rasmussen, D.N.; Nielsen, M.D.; Billestrup, N.; Grunnet, L.G.; Mandrup-Poulsen, T. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol. Med. 2011, 17, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Vasquez, D.S.; Ravnskjaer, K.; Denechaud, P.D.; Yu, R.T.; Alvarez, J.G.; Downes, M.; Evans, R.M.; Montminy, M.; Shaw, R.J. Class IIA histone deacetylases are hormone-activated regulators of foxo and mammalian glucose homeostasis. Cell 2011, 145, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yamada, T.; Matsumoto, M.; Kido, Y.; Hosooka, T.; Asahara, S.; Matsuda, T.; Ota, T.; Watanabe, H.; Sai, Y.; et al. Endoplasmic reticulum stress inhibits stat3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes 2012, 61, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Mosley, A.L.; Ozcan, S. The pancreatic duodenal homeobox-1 protein (PDX-1) interacts with histone deacetylases HDAC-1 and HDAC-2 on low levels of glucose. J. Biol. Chem. 2004, 279, 54241–54247. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of PDX1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. Foxp3+ regulatory t cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kolodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappab and pparalpha. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Menzel, T.; Luhrs, H.; Zirlik, S.; Schauber, J.; Kudlich, T.; Gerke, T.; Gostner, A.; Neumann, M.; Melcher, R.; Scheppach, W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 2004, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Castaner, O.; Elosua, R.; Pinto, X.; Estruch, R.; Salas-Salvado, J.; Corella, D.; Aros, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: A randomized controlled trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Paulionis, L.; Poon, T.; Lee, H.Y. The effects of almond consumption on fasting blood lipid levels: A systematic review and meta-analysis of randomised controlled trials. J. Nutr. Sci. 2016, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Oda, K.; Ros, E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch. Intern. Med. 2010, 170, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDLcholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Griel, A.E.; Kris-Etherton, P.M. Tree nuts and the lipid profile: A review of clinical studies. Br. J. Nutr. 2006, 96 (Suppl. 2), S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; West, S.G.; Kay, C.D.; Alaupovic, P.; Bagshaw, D.; Kris-Etherton, P.M. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: A dose-response study. Am. J. Clin. Nutr. 2008, 88, 651–659. [Google Scholar] [PubMed]
- Mukuddem-Petersen, J.; Oosthuizen, W.; Jerling, J.C. A systematic review of the effects of nuts on blood lipid profiles in humans. J. Nutr. 2005, 135, 2082–2089. [Google Scholar] [PubMed]
- Ostlund Jr, R.E. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. Alpha-linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, D.J. Dietary fatty acids, lipoproteins, and cardiovascular disease. Adv. Food Nutr. Res. 1992, 36, 253–351. [Google Scholar] [PubMed]
- Campos, H.; Dreon, D.M.; Krauss, R.M. Associations of hepatic and lipoprotein lipase activities with changes in dietary composition and low density lipoprotein subclasses. J. Lipid Res. 1995, 36, 462–472. [Google Scholar] [PubMed]
- Brousseau, M.E.; Ordovas, J.M.; Osada, J.; Fasulo, J.; Robins, S.J.; Nicolosi, R.J.; Schaefer, E.J. Dietary monounsaturated and polyunsaturated fatty acids are comparable in their effects on hepatic apolipoprotein mrna abundance and liver lipid concentrations when substituted for saturated fatty acids in cynomolgus monkeys. J. Nutr. 1995, 125, 425–436. [Google Scholar] [PubMed]
- Park, Y.; Carr, T.P. Unsaturated fatty acids and phytosterols regulate cholesterol transporter genes in Caco-2 and HepG 2 cell lines. Nutr. Res. 2013, 33, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D. Protein and atherosclerosis. J. Nutr. Sci. Vitaminol. 1990, 36, S35–S45. [Google Scholar] [CrossRef]
- Huff, M.W.; Carroll, K.K. Effects of dietary protein on turnover, oxidation, and absorption of cholesterol, and on steroid excretion in rabbits. J. Lipid Res. 1980, 21, 546–548. [Google Scholar] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Escola-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T. The cholesterol-lowering action of plant stanol esters. J. Nutr. 1999, 129, 2109–2112. [Google Scholar] [PubMed]
- Trautwein, E.A.; Duchateau, G.S.; Lin, Y.; Mel’nikov, S.M.; Molhuizen, H.O.; Ntanios, F.Y. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Technol. 2003, 105, 171–185. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Suzuki, H. Niemann-pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in Caco-2 cells. J. Pharmacol. Exp. Ther. 2007, 320, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Yu, L. Opposing gatekeepers of apical sterol transport: Niemann-pick C1-like 1 (NPC1L1) and atp-binding cassette transporters G5 and G8 (ABCG5/ABCG8). Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 18–29. [Google Scholar] [CrossRef] [PubMed]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Wong, W.T.; Guan, L.; Tian, X.Y.; Ma, K.Y.; Huang, Y.; Chen, Z.Y. Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis 2011, 219, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Brauner, R.; Johannes, C.; Ploessl, F.; Bracher, F.; Lorenz, R.L. Phytosterols reduce cholesterol absorption by inhibition of 27-hydroxycholesterol generation, liver X receptor alpha activation, and expression of the basolateral sterol exporter ATP-binding cassette A1 in Caco-2 enterocytes. J. Nutr. 2012, 142, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Born, E.; Mathur, S.N. Effect of micellar beta-sitosterol on cholesterol metabolism in Caco-2 cells. J. Lipid Res. 1997, 38, 348–360. [Google Scholar] [PubMed]
- Ho, S.S.; Pal, S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HEPG2 liver and Caco-2 intestinal cells. Atherosclerosis 2005, 182, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Pérez-Heras, A.; Ros, E. Dietary fibre, nuts and cardiovascular diseases. Br. J. Nutr. 2006, 96, S45–S51. [Google Scholar] [CrossRef]
- Lopez-Uriarte, P.; Bullo, M.; Casas-Agustench, P.; Babio, N.; Salas-Salvado, J. Nuts and oxidation: A systematic review. Nutr. Rev. 2009, 67, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Bibiloni, M.D.M.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.Á. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The predimed study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, R.L.; Etherton, T.D.; Pearson, T.A.; Harrison, E.H.; Kris-Etherton, P.M. Low fat and high monounsaturated fat diets decrease human low density lipoprotein oxidative susceptibility in vitro. J. Nutr. 2001, 131, 1758–1763. [Google Scholar] [PubMed]
- Berry, E.M.; Eisenberg, S.; Friedlander, Y.; Harats, D.; Kaufmann, N.A.; Norman, Y.; Stein, Y. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins—The jerusalem nutrition study. II. Monounsaturated fatty acids vs carbohydrates. Am. J. Clin. Nutr. 1992, 56, 394–403. [Google Scholar] [PubMed]
- Bonanome, A.; Pagnan, A.; Biffanti, S.; Opportuno, A.; Sorgato, F.; Dorella, M.; Maiorino, M.; Ursini, F. Effect of dietary monounsaturated and polyunsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modification. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 529–533. [Google Scholar] [CrossRef]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Imaizumi, K.; Sato, M.; Hirooka, Y.; Sakai, K.; Takeshita, A.; Kono, M. Serum lipid profiles in japanese women and men during consumption of walnuts. Eur. J. Clin. Nutr. 2002, 56, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.; Merlos, M.; Zambon, D.; Rodriguez, C.; Sabate, J.; Ros, E.; Laguna, J.C. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HEPG2 cells. J. Lipid Res. 2001, 42, 2069–2076. [Google Scholar] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Chen, C.Y.; Blumberg, J.B. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J. Agric. Food Chem. 2008, 56, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Chung, S.K.; Blumberg, J. Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. J. Nutr. Biochem. 2007, 18, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Jambazian, P.R.; Haddad, E.; Rajaram, S.; Tanzman, J.; Sabate, J. Almonds in the diet simultaneously improve plasma alpha-tocopherol concentrations and reduce plasma lipids. J. Am. Diet. Assoc. 2005, 105, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, M.; Moreno, J.J. Beta-sitosterol modulates antioxidant enzyme response in raw 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Segura, R.; Javierre, C.; Lizarraga, M.A.; Ros, E. Other relevant components of nuts: Phytosterols, folate and minerals. Br. J. Nutr. 2006, 96 (Suppl. 2), S36–S44. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human ldl resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [PubMed]
- Jia, X.; Li, N.; Zhang, W.; Zhang, X.; Lapsley, K.; Huang, G.; Blumberg, J.; Ma, G.; Chen, J. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr. Cancer 2006, 54, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Stonehouse, W.; Mukuddem-Petersen, J.; van der Westhuizen, F.H.; Hanekom, S.M.; Jerling, J.C. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur. J. Nutr. 2007, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.L.; Blake, R.J.; Wills, R.B.; Clayton, E.H. Macadamia nut consumption modulates favourably risk factors for coronary artery disease in hypercholesterolemic subjects. Lipids 2007, 42, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jia, X.; Chen, C.Y.; Blumberg, J.B.; Song, Y.; Zhang, W.; Zhang, X.; Ma, G.; Chen, J. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J. Nutr. 2007, 137, 2717–2722. [Google Scholar] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Blumberg, J. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J. Nutr. 2008, 138, 908–913. [Google Scholar] [PubMed]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabate, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Jambazian, P.; Karunia, M.; Tanzman, J.; Sabaté, J. A pecan-enriched diet increases γ-tocopherol/cholesterol and decreases thiobarbituric acid reactive substances in plasma of adults. Nutr. Res. 2006, 26, 397–402. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.Y.; Yeum, K.J.; Matthan, N.R.; Lichtenstein, A.H.; Blumberg, J.B. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: A randomized, cross-over pilot study. Nutr. J. 2010, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sanchez-Gonzalez, C.; Vallverdu-Queralt, A.; Simal-Gandara, J.; Lamuela-Raventos, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Khanabadi, B.A.; Mozaffari-Khosravi, H.; Parsaeyan, N. Effects of almond dietary supplementation on coronary heart disease lipid risk factors and serum lipid oxidation parameters in men with mild hyperlipidemia. J. Altern. Complement. Med. 2010, 16, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Liu, Y.H.; Chen, C.M.; Chang, W.H.; Chen, C.Y. The effect of almonds on inflammation and oxidative stress in chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. Irs-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Malik, V.S.; Keum, N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between nut consumption and inflammatory biomarkers. Am. J. Clin. Nutr. 2016, 104, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Arpon, A.; Riezu-Boj, J.I.; Milagro, F.I.; Razquin, C.; Martinez-Gonzalez, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fito, M.; Ros, E.; et al. Adherence to mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 2017, 73, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar] [PubMed]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [PubMed]
- Rajaram, S.; Connell, K.M.; Sabate, J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: A randomised, controlled, crossover study. Br. J. Nutr. 2010, 103, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Salas-Salvado, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bullo, M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effect of almond supplementation on glycemia and cardiovascular risk factors in Asian Indians in North India with type 2 diabetes mellitus: A 24-week study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of N-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [PubMed]
- Yu, Y.M.; Wang, Z.H.; Liu, C.H.; Chen, C.S. Ellagic acid inhibits IL-1beta-induced cell adhesion molecule expression in human umbilical vein endothelial cells. Br. J. Nutr. 2007, 97, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Yan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Xun, P.; He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.Y.; van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [PubMed]
- Gharibeh, M.Y.; Al Tawallbeh, G.M.; Abboud, M.M.; Radaideh, A.; Alhader, A.A.; Khabour, O.F. Correlation of plasma resistin with obesity and insulin resistance in type 2 diabetic patients. Diabetes Metab. 2010, 36, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, D.Y.; Cao, J.; He, Z.Y.; Zhu, B.P.; Long, M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin. Med. Sci. J. 2009, 24, 161–166. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Y.; Li, F.; He, J.; Ding, H.; Yan, L.; Cheng, H. Resistin induces insulin resistance by both AMPK-dependent and ampk-independent mechanisms in HEPG2 cells. Endocrine 2009, 36, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Hu, F.B.; Rifai, N.; Manson, J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004, 291, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Laight, D.W.; Carrier, M.J.; Anggard, E.E. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc. Res. 2000, 47, 457–464. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 2009, 32 (Suppl. 2), S314–S321. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, S.; Higano, S.; Nishimura, R. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar]
- Federici, M.; Pandolfi, A.; De Filippis, E.A.; Pellegrini, G.; Menghini, R.; Lauro, D.; Cardellini, M.; Romano, M.; Sesti, G.; Lauro, R.; et al. G972R IRS-1 variant impairs insulin regulation of endothelial nitric oxide synthase in cultured human endothelial cells. Circulation 2004, 109, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, E.L.; Cho, H.; Birnbaum, M.J. Role of AKT/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002, 13, 444–451. [Google Scholar] [CrossRef]
- Montagnani, M.; Ravichandran, L.V.; Chen, H.; Esposito, D.L.; Quon, M.J. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol. Endocrinol. 2002, 16, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Wang, K.Q.; Zhou, G.M.; Zuo, J.; Ge, J.B. Endothelin-1 promoted proliferation of vascular smooth muscle cell through pathway of extracellular signal-regulated kinase and cyclin D1. Acta Pharmacol. Sin. 2003, 24, 563–568. [Google Scholar] [PubMed]
- Eringa, E.C.; Stehouwer, C.D.; van Nieuw Amerongen, G.P.; Ouwehand, L.; Westerhof, N.; Sipkema, P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Libby, P.; Peng, H.B.; Thannickal, V.J.; Rajavashisth, T.B.; Gimbrone, M.A., Jr.; Shin, W.S.; Liao, J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 1995, 96, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Adams, H.R.; Myers, J.L. Effects of cytokines tumor necrosis factor [alpha] and interleukin 1 [beta] on endotoxin-mediated inhibition of endothelium-derived relaxing factor bioactivity and nitric oxide production in vascular endothelium. Shock 1994, 1, 73–78. [Google Scholar] [CrossRef]
- Grau, G.E.; Lou, J. Tnf in vascular pathology: The importance of platelet-endothelium interactions. Res. Immunol. 1993, 144, 355–363. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Massaro, M.; Bonfrate, C.; Siculella, L.; Maffia, M.; Nicolardi, G.; Distante, A.; Storelli, C.; De Caterina, R. Oleic acid inhibits endothelial activation: A direct vascular antiatherogenic mechanism of a nutritional component in the Mediterranean diet. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, M.; Hattori, S.; Kasai, K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia 2003, 46, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Vamvini, M.T.; Chamberland, J.P.; Sweeney, L.L.; Brennan, A.M.; Magkos, F.; Mantzoros, C.S. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein a concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: Data from a double-blinded, randomized, placebo-controlled study. Metabolism 2012, 61, 577–582. [Google Scholar] [PubMed]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: A randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cortés, B.; Núñez, I.; Cofán, M.; Gilabert, R.; Pérez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kasliwal, R.R.; Bansal, M.; Mehrotra, R.; Yeptho, K.P.; Trehan, N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition 2015, 31, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.; Valacchi, G.; Pagnin, E.; Shao, Q.; Gross, H.B.; Calo, L.; Yokoyama, W. Walnuts reduce aortic ET-1 mrna levels in hamsters fed a high-fat, atherogenic diet. J. Nutr. 2006, 136, 428–432. [Google Scholar] [PubMed]
- Gornik, H.L.; Creager, M.A. Arginine and endothelial and vascular health. J. Nutr. 2004, 134, 2880S–2887S, discussion 2895S. [Google Scholar] [PubMed]
- Shechter, M.; Sharir, M.; Labrador, M.J.; Forrester, J.; Silver, B.; Bairey Merz, C.N. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Pineo, A.; Belvedere, M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magn. Res. 2010, 23, 131–137. [Google Scholar]
- Wells, B.J.; Mainous, A.G., 3rd; Everett, C.J. Association between dietary arginine and C-reactive protein. Nutrition 2005, 21, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Porat, R.; Rosenschein, U.; Keren, G.; Roth, A.; Laniado, S.; Miller, H. Clinical and inflammatory effects of dietary l-arginine in patients with intractable angina pectoris. Am. J. Cardiol. 1999, 83, 1488–1490. [Google Scholar] [CrossRef]
- Adams, M.R.; McCredie, R.; Jessup, W.; Robinson, J.; Sullivan, D.; Celermajer, D.S. Oral l-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis 1997, 129, 261–269. [Google Scholar] [CrossRef]
- Yin, W.-H.; Chen, J.-W.; Tsai, C.; Chiang, M.-C.; Young, M.S.; Lin, S.-J. l-arginine improves endothelial function and reduces ldl oxidation in patients with stable coronary artery disease. Clin. Nutr. 2005, 24, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvado, J.; Guasch-Ferre, M.; Humphries, K.; Sarrafzadegan, N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015, 101, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvado, J.; Covas, M.I.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Effect of the mediterranean diet on blood pressure in the predimed trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenech, M.; Roman, P.; Lapetra, J.; Garcia de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Mediterranean diet reduces 24-h ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remon, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The predimed randomized trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
| Almond | Walnut | Hazelnut | Macadamia | Pecan | Pistachio | Brazil Nut | Cashew Nut | Peanut | Pine Nut | Chest Nut | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | 579 | 654 | 628 | 718 | 691 | 560 | 659 | 553 | 567 | 673 | 213 |
| Carbohydrate (g) | 21.55 | 13.71 | 16.7 | 13.82 | 13.86 | 27.17 | 11.74 | 30.19 | 16 | 13.08 | 45.54 |
| Protein (g) | 21.15 | 15.23 | 14.95 | 7.91 | 9.17 | 20.16 | 14.32 | 18.22 | 26 | 13.69 | 2.42 |
| Lysine (g) | 0.568 | 0.424 | 0.42 | 0.018 | 0.287 | 1.138 | 0.49 | 0.928 | 0.926 | 0.54 | 0.143 |
| Arginine (g) | 2.465 | 2.278 | 2.211 | 1.402 | 1.177 | 2.134 | 2.14 | 2.123 | 3.085 | 2.413 | 0.173 |
| Total fat (g) | 49.93 | 65.21 | 60.75 | 75.77 | 71.97 | 45.32 | 67.1 | 43.85 | 49 | 68.37 | 2.26 |
| Saturated fat (g) | 3.8 | 6.126 | 4.464 | 12.061 | 6.18 | 5.907 | 16.134 | 7.783 | 7 | 4.89 | 0.425 |
| MUFA (g) | 31.55 | 8.933 | 45.652 | 58.877 | 40.801 | 23.257 | 23.879 | 23.797 | 24 | 18.76 | 0.78 |
| PUFA (g) | 12.329 | 47.174 | 7.92 | 1.502 | 21.614 | 14.38 | 24.399 | 7.845 | 16 | 34.07 | 0.894 |
| Total Fiber (g) | 12.5 | 6.7 | 9.7 | 8.6 | 9.6 | 10.6 | 7.5 | 3 | 8.5 | 3.7 | 8.1 |
| Folate (μg) | 44 | 98 | 113 | 11 | 22 | 51 | 22 | 25 | 240 | 34 | 62 |
| Calcium (mg) | 269 | 98 | 114 | 85 | 70 | 105 | 160 | 37 | 92 | 16 | 27 |
| Magnesium (mg) | 270 | 158 | 163 | 130 | 121 | 121 | 376 | 292 | 168 | 251 | 32 |
| Sodium (mg) | 1 | 2 | 0 | 5 | 0 | 1 | 3 | 12 | 18 | 2 | 3 |
| Potassium (mg) | 733 | 441 | 680 | 368 | 410 | 1025 | 659 | 660 | 705 | 597 | 518 |
| Copper (mg) | 1.031 | 1586 | 1725 | 0.756 | 1.2 | 1.3 | 1.743 | 2195 | 1.144 | 1.324 | 0.447 |
| Iron (mg) | 3.71 | 2.91 | 4.7 | 3.69 | 2.53 | 3.92 | 2.43 | 6.68 | 4.58 | 5.53 | 1.01 |
| Zinc (mg) | 3.12 | 3.09 | 2.45 | 1.3 | 4.53 | 2.2 | 4.06 | 5.78 | 3.27 | 6.45 | 0.52 |
| Selenium (μg) | 4.1 | 4.9 | 2.4 | 3.6 | 3.8 | 7 | 1917 | 19.9 | 7.2 | 0.7 | NA |
| α-tocopherol (mg) | 25.63 | 0.7 | 15.03 | 0.54 | 1.4 | 2.86 | 5.65 | 0 | 8.33 | 9.33 | NA |
| β-tocopherol (mg) | 0.23 | 0.15 | 0.33 | 0 | 0.39 | 0 | 0.01 | 0.03 | NA | 0 | NA |
| γ-tocopherol (mg) | 0.07 | 20.83 | 0 | 0 | 24.44 | 20.41 | 9.56 | 5.31 | NA | 11.15 | NA |
| δ-tocopherol (mg) | 0.07 | 1.89 | 0 | 0 | 0.47 | 0.8 | 0.63 | 0.36 | NA | 0 | NA |
| Total phytosterol (mg) | ~198 | ~110.2 | ~122 | ~116 | ~158.7 | ~214 | ~123.8 | ~151 | NA | 236.1 | 22 |
| Stigmasterol | 4 | 0 | 1 | 0 | 3 | 5 | 6 | 0 | 0 | - | |
| Campesterol | 5 | 5 | 7 | 8 | 6 | 10 | 2 | 9 | 20 | - | |
| B-sitosterol | 130 | 87 | 102 | 108 | 117 | 198 | 64 | 113 | 132 | - | |
| δ 5-avenasterol | 21 | 7.3 | 2.6 | - | 14.3 | - | 19.7 (δ avenasterol + B sitostanol) | 14 | 40.1 | - | |
| B-sitostanol | 4 | - | 3.9 | - | - | - | - | 5.9 | - | ||
| Campestanol | 2 | 2.3 | 3 | - | 2.8 | - | - | 2 | 3.9 | - | |
| Others | 32 | 8.6 | 2.5 | - | 15.6 | - | 31.8 | 13 | 34.2 | - | |
| Total polyphenol (mg) # | 287 | 1576 | 687 | 126 | 1284 | 867 | 224 | 232.9 | NA | NA | NA |
| Total polyphenol (mg) $ | 212.9 ± 12.3 | 1580.5 ± 58 | 314.8 ± 47.3 | 497.8 ± 52.6 | 1463.9 ± 32.3 | 571.8 ± 12.5 | 169.2 ± 14.6 | 316.4 ± 7.0 | 645.9 ± 47 | 152.9 ± 14.1 | NA |
| Flavonoids (mg) $ | 93.5 ± 10.8 | 744.8 ± 93.3 | 113.7 ± 30.2 | 137.9 ± 9.9 | 704.7 ± 29.5 | 143.3 ± 18.7 | 107.8 ± 6.0 | 63.7 ± 2.1 | 189.8 ± 13.1 | 45.0 ± 5.4 | NA |
| Ellagitannins (mg) ‡ | ND | 823 ± 59 | ND | ND | 301 ± 7 | ND | ND | ND | ND | NA | 149 ± 3 |
| Proanthocyanidins (mg) # | 176 | 60 | 491 | NA | 477 | 226 | NA | 2 | NA | NA | 0 |
| Carotenoids (μg) | 2 | NA | 106 | NA | 55 | 332 | 0 | NA | NA | NA | NA |
| Lutein + zeaxanthin (μg) | 1 | 9 | 92 | 0 | 17 | 2903 | 0 | 22 | 0 | 9 | NA |
| Description | Reference | Subjects | Study Design/Period | Nut Type | Diet Intervention | Results |
|---|---|---|---|---|---|---|
| Meta-analysis of 12 RCTs for glycemic control | Viguiliouk et al., 2014 [20] | Subjects with T2DM | RCTs/≥3-week follow-up period | Almonds, pistachios, walnuts, pecans, hazelnuts, peanuts, cashews, macadamias | Diet supplemented with tree nuts vs. isocaloric diet without tree nuts | ↓ HbA1c (p = 0.0003), ↓ fasting glucose, |
| ↔ fasting insulin, ↔ HOMA-IR after consumption of tree nuts at a median dose of 56 g/day | ||||||
| Chronic studies that were not included in meta-analysis of Viguiliouk et al., 2014 [20] | Scott et al., 2003 [25] | 17 subjects with IGT (n = 10) or T2DM (n = 7) | Parallel, randomised/42 weeks | Almonds | Almond-enriched diet (High Protein-High MUFA diet; 25% of energy from protein, 40% fat, and 22% MUFAs) vs. AHA diet (15% of energy from protein, 30% fat, and 15% MUFAs) | ↑ glucose control |
| ↔ TG, ↔ LDL-C, ↔ fasting glucose | ||||||
| Wien et al., 2003 [26] | 65 overweight and obese subjects | Randomised prospective/24 weeks | Almonds | Formula-based low-calorie diet enriched with 84 g/day of almonds vs. complex carbohydrates matching calories and protein | ↓ 62% body weight/BMI, | |
| ↓ 50% waist circumference, | ||||||
| ↓ 56% greater reduction in fat mass compared with the complex carbohydrate diet | ||||||
| ↓ glucose, ↓ insulin, ↓ HOMA-IR, ↓ TC, ↓ TG, ↓ LDL-C, ↓ LDL-C/HDL-C in both diets | ||||||
| Sari et al., 2010 [24] | 32 healthy young men | Dietary intervention/8 weeks | Pistachios | Subjects consumed a Mediterranean diet for 4 weeks and in the next 4 weeks they supplemented the Mediterranean diet with pistachios for 4 weeks replacing 32% of the energy obtained from MUFAs in the control diet (20% of energy) | ↓ glucose levels (−8.8 ± 8.5% p < 0.001) | |
| ↓ TC (p < 0.001), ↓ LDL-C (p < 0.001), ↓ triacylglycerol, ↔ HDL-C, | ||||||
| ↓ TC-C/HDL-C, ↓ LDL-C/HDL-C (p < 0.001), | ||||||
| ↑ endothelium-dependent vasodilation (p = 0.002) | ||||||
| ↓ lipid hydroperoxide (p < 0.001), ↓ MDA (p < 0.001), ↓ IL-6 (p < 0.001), | ||||||
| ↑ SOD (p < 0.001), | ||||||
| ↔ hs-CRP, ↔ TNF-α | ||||||
| compared with a Mediterranean diet | ||||||
| Wien et al., 2010 [36] | 65 subjects with prediabetes | Randomized parallel trial/16 weeks | Almonds | ADA diet containing 20% of energy from almonds (60 g/day of pre-packaged raw or dry roasted almonds) vs. almond-free control diet | ↓ fasting insulin (p = 0.002), ↓ HOMA-IR (p = 0.007), ↓ HOMA-B (p = 0.001), ↔ fasting glucose, ↓. LDL-C, ↔ BMI, | |
| ↓ weight, ↔ waist circumference | ||||||
| Parham et al., 2014 [23] | 48 subjects with T2DM | Double-blind, randomized, placebo-controlled, crossover/12 weeks | Pistachios | 25 g pistachio nuts twice a day as a snack vs. control diet without nuts | ↓ HbA1c (−0.4%; p ≤ 0.001), ↓ fasting blood glucose (−16 mg/dL; p ≤ 0.001), | |
| ↔ BMI, ↔ BP, ↔ HOMA-IR, ↔ hs-CRP | ||||||
| Sauder et al., 2015 [22] | 30 subjects with well-controlled T2DM | Randomized, crossover, controlled/4 weeks | Pistachios | Healthy diet with pistachios contributing 20% of total energy. Low-fat or fat-free snacks (e.g., pretzels) in the control diet were substituted with roasted pistachios providing 20% of daily energy (59 to 128 g/day of pistachios). Half of amount of pistachio was ingested unsalted vs. AHA Therapeutic Lifestyle Changes diet (26.9% total fat, 6.7% SFA, 186 mg/day cholesterol). | ↓ fructosamine, ↓ TC, ↓ ratio of TC to HDL-C, ↓ TG (p = 0.003), ↔ HbA1c, | |
| ↔ fasting glucose, ↔ insulin, ↔ hs-CRP, | ||||||
| ↔ ICAM, ↔ VCAM, ↔ endothelial function | ||||||
| Zibaeenezhad et al., 2016 [21] | 100 subjects with T2DM | Randomized control/3 months | Walnut oil | Walnut oil (15 g/day) group (n = 50) vs. a control group without any interventions (n = 50) | ↓ HbA1c by 8 ± 22% (p = 0.005) and | |
| ↓ fasting glucose by 8 ± 17% (p = 0.001) within the walnut group. | ||||||
| But, ↔ HbA1c, ↔ fasting glucose compared with the control. | ||||||
| ↔ body weight, BMI, BP in the two groups | ||||||
| Acute studies that were not included in meta-analysis | Johnston et al., 2005 [30] | 11 healthy subjects | Randomised crossover | Peanuts | 6 meals (bagel and juice meal, bagel and juice meal + vinegar, bagel and juice meal + peanut, chicken and rice meal, chicken and rice meal + vinegar, and chicken and rice meal + peanut) were compared | ↓ glucose response in the 60-min (p < 0.05) following the ingestion of vinegar or peanuts with bagel and juice meal |
| Jenkins et al., 2006 [32] | 15 healthy subjects | Meals consumed in random order on separate days, 4-h postprandial test | Almonds | 3 test meals; almonds (60 g) + bread (97 g), parboiled rice meal (68 g cheese + 14 g butter + 60 g parboiled rice) and mashed potato meal (62 g cheese + 16 g butter + 68 g mashed potatoes) vs. 2 bread control meals | ↓ postprandial glucose and insulin (p < 0.001) responses | |
| ↑ protein thiol | ||||||
| ↔ total antioxidant capacity compared with other test meals | ||||||
| Josse et al., 2007 [33] | 9 healthy subjects | Meals consumed in random order on separate days, 2-h postprandial test | Almonds | White bread + 0 g almond, white bread + 30 g almond, white bread + 60 g almond, white bread + 90 g almond, | ↓ meal glycemic index in a dose-dependent manner (90 g almond + white bread > 60 g almond + white bread > 30 g almond + white bread; p = 0.001) | |
| Each meal contained 50 g CHO from white bread | ||||||
| Kendall et al., 2011 [35] | 14 normoglycemic subjects and 10 subjects with T2DM | Meals consumed in random order on separate days, 2-h postprandial test | Mixed nuts | Mixed nuts (30, 60 and 90 g) were consumed with white bread | ↓ postprandial glycemic responses | |
| Each meal contained 50 g CHO from white bread | ||||||
| Kendall et al., 2011 [27] | 10 healthy adults | Two acute tests; each test consisting of 2-h postprandial test | Pistachios | <Study 1> 8 test meals: white bread (50 g CHO), 28 g pistachio, 56 g pistachio, 84 g pistachio, white bread + 28 g pistachios, white bread + 56 g pistachios and white bread + 84 g pistachios, aiming to assess the dose-response effect of 28, 56 and 84 g pistachios ingested alone or with white bread. <Study 2> 7 test meals: white bread (50 g CHO), parboiled rice, pasta and instant mashed potatoes and parboiled rice + 56 g pistachios, pasta + 56 g pistachios instant mashed potatoes + 56 g pistachios, aiming to assess effective 56 g of pistachio on glycemic response with different types of CHO foods | Study 1: relative glycemic responses; for 28 g, 56 g and 84 g pistachios were 5.7%, 3.8% and 9.3% respectively compared to white bread, ↓ relative glycemic responses; in a dose-dependent manner with 89% (p = 0.1), 67% (p = 0.009) and 52% (p < 0.001) for 28 g, 56 g and 84 g pistachios added to white bread respectively Study 2: ↓ relative glycemic responses; after adding 56 g pistachios to rice, pasta and instant mashed potatoes with 59% (p = 0.02), 56% (p = 0.025) and 87% (p = 0.06) respectively, compared with 73%, 95%, 109% | |
| Mori et al., 2011 [31] | 14 subjects with IGT | Randomized, 5-arm, crossover | Almonds | Whole almonds into breakfast vs. almond butter into breakfast vs. defatted almond flour into breakfast vs. almond oil into breakfast vs. no almonds into breakfast | ↓ postprandial glycemia and ↑ satiety following whole almonds ↓ the second-meal NEFA response following whole almonds or almond oil ↔ GLP-1 between meals | |
| All breakfast meals were matched for 75 g carbohydrate | ||||||
| Reis et al., 2013 [29] | 15 obese women with a high T2DM risk | Randomised cross-over | Peanuts | Consumption of a 75 g CHO-matched breakfast containing 42.5 g of whole peanuts without skins or peanut butter or no peanuts to meal and then consumption of standard lunch, aiming to assess first- and second meal responses | ↓ NEFA iAUC (0–240 min), ↓ glucose iAUC (240–490 min), ↑ insulin (120–370 min) ↑ gut satiety hormones (PYY, GLP-1 and CCK), ↓ desire to eat following peanut butter breakfast compared with no peanut breakfast | |
| Kendall et al., 2014 [28] | 20 subjects with metabolic syndrome | Randomized crossover | Pistachios | 5 test meals: | ↓ glucose response ↑ GLP-1 | |
| - 3 meals containing 50 g available CHO: white bread, white bread, butter and cheese, and white bread and pistachios | ||||||
| - 2 meals containing12 g available CHO: white bread, and pistachios | ||||||
| Crouch et al., 2016 [34] | 20 subjects with prediabetes or isolated 1-h postprandial hyperglycemia | Almonds | one-half ounce (14.2 g) dry-roasted almond preload vs. without the almond preload. | ↓ glucose 1 h after a 75-g glucose challenge (p < 0.001). |
| Reference | Subjects | Study Design/Period | Nut Type | Diet Intervention | Results |
|---|---|---|---|---|---|
| Spiller et al., 1992 [64] | 26 subjects with hypercholesterolemia | Pre-post supplemental, dietary advice/9 weeks | Almonds | Raw almonds (100 g/day; 34 g/day MUFA, 12 g/day PUFA 6 g/day SFA) to baseline diet | ↔ body weight, ↑ 81 kcal/day energy intake,↓ LDL-C, ↓ TC (p < 0.001), ↔ HDL-C |
| Abbey et al., 1994 [53] | 16 male health subjects | Pre-post consecutive supplemental, dietary advice/9 weeks | Almonds, Walnuts | In the first 3-week period, addition of raw peanuts (50 g/day), coconut cubes (40 g/day), and a coconut confectionary bar (50 g/day), to Australian diet (reference diet) | ↔ body weight, ↔ energy intake ↓ LDL-C, ↓ TC, ↔ HDL-C compared with reference diet |
| During the following 3 weeks, 84 g/day almonds added to Australian diet | |||||
| During the final 3-week period, 68 g/day walnuts added to Australian diet | |||||
| Colquhoun et al., 1996 [76] | 14 subjects with hypercholesterolemia | Randomised crossover, pre-post, dietary advice/4 weeks | Macadamias | Diet enriched with macadamia (40% energy as fat, 20% energy from macadamia nuts) vs. a high-complex-carbohydrate diet | ↔ body weight, ↔ energy intake |
| ↓ LDL-C (p < 0.01), ↓ TC (p < 0.01) on both diets compared with baseline. | |||||
| ↔ HDL-C on both diets | |||||
| Spiller et al., 1998 [63] | 45 subjects with hypercholesterolemia | Randomised parallel arm, dietary advice/4 weeks | Almonds | Almond-based diet vs. olive oil-based diet vs. dairy-based diet | ↔ body weight, ↔ energy intake |
| ↓ TC (p < 0.001), ↓ LDL-C (p < 0.001) compared with control diets | |||||
| Three diets were matched for total fat | ↓ TC, ↓ LDL-C (p < 0.001), ↓ TC:HDL ratio (p < 0.001), ↔ HDL-C within the almond group | ||||
| Chisholm et al., 1998 [67] | 21 subjects with hypercholesterolemia | Randomised crossover, dietary advice/4 weeks | Walnuts | 20% energy of low-fat diet replaced with 78 g/day walnuts vs. low-fat diet | ↔ body weight, ↔ energy intake, ↓ TC, ↓ LDL-C, ↑ HDL-C on both diets compared with baseline. |
| ↓ apo B on the walnut diet compared with low-fat diet | |||||
| Durak et al., 1999 [77] | 30 healthy subjects | Pre-post supplemental, dietary advice | Hazelnuts | 1 g/kg body weight/day of hazelnuts supplemented with a habitual diet vs. baseline | ↑ 0.5 kg body weight, ↓ TC (p < 0.005), ↓ LDL-C (p < 0.0005), |
| ↑ HDL-C, ↑ TG (p < 0.001), ↑ HDL-C/LDL-C,(p <,0.0005) | |||||
| ↓ MDA(p < 0.0005), ↑AOP (p < 0.0005) after hazelnut supplementation | |||||
| Edwards et al., 1999 [72] | 10 subjects with hypercholesterolemia | Randomised, controlled, crossover, dietary advice/3 weeks | Pistachios | 20% energy of habitual diet replaced with pistachios vs. control diet | ↔Body weight, ↔ BP, ↔ energy intake, ↓ TC, ↓ LDL-C, ↓ TC/HDL (p < 0.01), ↑ HDL-C, ↔ TG |
| Morgan et al., 2000 [70] | 19 subjects with normal lipid levels | Randomised parallel arm, pre-post, supplemental/8 weeks | Pecans | 68 g/day pecans + self-selected diet vs. self-selected diet without nuts | ↔ body weight, ↑ 71 kJ energy intake |
| ↓ TC, ↓ HDL-C | |||||
| Zambone et al., 2000 | 49 subjects with hypercholesterolemia | Randomised crossover, dietary advice/6 weeks | Walnuts | 18% energy of habitual diet replaced with walnuts vs. Mediterranean diet | ↔ body weight, ↔ energy intake |
| ↓ TC (p < 0.001), ↓ LDL-C (p < 0.00), ↓ lipoprotein (a) compared with the Mediterranean diet, | |||||
| Curb et al., 2000 [75] | 30 healthy subjects | Randomized crossover/30 days | Macadamias | 3 dietary periods; American diet high in saturated fat (37% energy from fat) vs. AHA Step 1 diet (30% energy from fat) vs. MUFA diet rich in macadamia (37% energy from fat) | ↔ body weight, ↑ energy intake |
| ↓ TC, ↓ LDL-C after macadamia diet compared with American diet | |||||
| Almario et al., 2001 [52] | 18 subjects (7 men and 16 postmenopausal women) with dyslipidaemia | Pre-post consecutive supplemental, dietary advice/6 weeks | Walnuts | 48 g/day walnuts added to a habitual diet vs. | ↔ body weight, ↑ energy intake |
| 48 g/day walnuts added to a low-fat diet vs. | ↓ TC after walnut plus a low-fat diet compared with habitual diet or low-fat diet | ||||
| habitual diet vs. low-fat diet | ↓ LDL-C (p < 0.01) after walnut plus a low-fat diet compared with a low-fat diet | ||||
| McManus et al., 2001 [79] | 101 overweight subjects | Randomized controlled/18 months | Peanuts, almonds, cashews, hazelnuts, macadamias, pecans, pistachios, walnuts | Moderate fat low energy diet (fat: 35% of energy; predominantly PUFAs; nut-enriched diet) vs. low fat, low-energy diet (fat: 20% of energy) | ↓ 4.1 kg body weight, ↓ 1.6 kg/m2 BMI, ↓ 6.9 cm waist circumference in moderate fat group, while ↑ 2.9 kg body weight, ↑ 1.4 kg/m2 BMI, ↑ 2.6 cm waist circumference in low fat group |
| Fraser et al., 2002 [50] | 81 male and female subjects | Randomized crossover/6 months | Almonds | 42–70 g/day almond supplementation (320 kcal/day) vs. without almond supplementation | ↑ 0.40 kg body weight |
| Jenkins et al., 2002 [62] | 27 subjects with hypercholesterolemia | Randomised crossover, dietary advice/4 weeks | Almonds | 3 isoenergetic (mean 423 kcal/day) supplement periods each for 4 weeks; - full-dose almonds (73 g/day), - half-dose almonds plus half-dose muffins, - full-dose muffins (control) Supplements provided 22.2% of energy | ↔ body weight, ↔ energy intake |
| ↓ LDL-C (p < 0.001), ↓ LDL-C:HDL-C (p < 0.001) on both almonds | |||||
| ↓ oxidised LDL (p < 0.001), ↓ lipoprotein (a) on full -dose almonds | |||||
| ↔ pulmonary nitric oxide between control, half-dose, and full-dose almond diet | |||||
| Hyson et al., 2002 [65] | 22 subjects with normal lipid levels | Randomised crossover, pre-post, supplemental/6 weeks | Almonds | 50% of fat energy of habitual diet replaced with almonds (~66 ± 5 g/day), vs. 50% of fat energy of habitual diet replaced with almond oil (~35 ± 2 g/day) | ↔ body weight, ↔ energy intake |
| ↓ TC, ↓ LDL-C, ↓ TG, ↑ HDL compared with baseline | |||||
| Morgan et al., 2002 [69] | 42 subjects with borderline high total cholesterol | Randomised crossover, dietary advice/6 weeks | Walnuts | 64 g/day of walnuts added to low-fat, low-cholesterol diet vs. low-fat, low-cholesterol diet | ↔ body weight, ↔ energy intake |
| ↓ TC, ↓ LDL-C, ↓ TG, ↑ HDL-C | |||||
| ↔ homocysteine ,↓ tPA, ↓PAI-1 | |||||
| Lovejoy et al., 2002 [37] | 20 healthy subjects | Pre-post supplemental study, dietary advice/4 weeks | Almonds | 100 g/day of almond added to habitual diet vs. baseline | ↑ body weight (↑ 0.9 kg; male, ↑ 0.3 kg: female), ↑ ~51 kJ energy intake, ↓ LDL-C (p = 0.0004), ↔ insulin sensitivity compared with baseline |
| Alper et al., 2002 [49] | 15 healthy subjects with normal weight | Crossover, 3 treatment phases (free-feeding, addition and substitution)/30 weeks | Peanuts | In the free-feeding phase, 50% of dietary fat energy from peanuts for 8 weeks with no restriction of the background diet. No dietary advice given. | ↔ body weight during the substitution phase |
| In the addition phase, peanuts added to their habitual diet for 3 weeks with dietary instructions | ↑ 1 kg body weight during the free-feeding phase | ||||
| In a substitution phase, an equivalent quantity of fat from peanuts substituted for fat in the diet for 8 weeks. Peanuts consumed at average of 89 ± 21 g/day equivalent to 2113 ± 494 kJ/day (505 ± 118 kcal/day) during the 3 treatment phases. 50% dietary fat energy provided by peanuts. | ↑ resting energy expenditure by 11% after adjustment for changes in body weight after peanut consumption for 19 weeks (p < 0.01) | ||||
| Wien et al., 2003 [26] | 65 overweight and obese subjects | Randomised prospective/24 weeks | Almonds | Formula-based low-calorie diet enriched with 84 g/day of almonds vs. complex carbohydrates matching calories and protein | ↓ 62% body weight/BMI, |
| ↓ 50% waist circumference, | |||||
| ↓ 56% greater reduction in fat mass compared with the complex carbohydrate diet | |||||
| ↓ glucose, ↓ insulin, ↓ HOMA-IR,↓ TC, ↓ TG, ↓ LDL-C, ↓ LDL-C/HDL-C in both diets | |||||
| Pelkman et al., 2004 [80] | 53 overweigh and obese subjects | Parallel-arm/10 weeks (6 weeks for weight loss and additional 4-week for weigh maintenance) | Peanuts | Moderate-fat (33% of energy) diet vs. low-fat (18% of energy) diet | ↓ 1.2 ± 0.05 kg/week body weight within the moderate-fat group |
| ↓ 1.09 ± 0.06 kg/week body weight within the low-fat diet groups | |||||
| No difference in weight loss between two groups. | |||||
| ↔ HDL-C, ↓ triacylglycerol, | |||||
| non-HDL C/HDL-C within the moderate-fat diet group for 6 weeks | |||||
| ↑ triacylglycerol, ↓ HDL-C, ↔ non-HDL-C/HDL-C within the low-fat diet group for 6 weeks | |||||
| Sabate et al., 2005 [66] | 90 healthy subjects | Randomized cross-over/6 months | Walnuts | 28–56 g/day walnut-supplementation (12% energy intake) vs. control diet without nuts | ↑ energy intake (133 kcal), ↑ 0.4 kg body weight |
| Kocyigit et al., 2006 [71] | 44 health subjects | Randomised parallel arm/3 weeks | Pistachios | 20% energy of regular diet replaced with pistachios vs. regular diet | ↔ body weight, ↔ energy intake |
| ↓ TC, ↓ MDA, ↓ TC/HDL-C (p < 0.001) | |||||
| ↓ LDL-C/HDL-C (p < 0.01), ↑ HDL-C (p < 0.001), ↑AOP, ↑AOP/MDA | |||||
| Hollis et al., 2007 [51] | 20 healthy female subjects | Randomised crossover, no dietary advice/10 weeks | Almonds | 1440 kJ portion of raw, unsalted almonds added to a habitual diet vs. habitual diet | ↔ body weight, ↔ energy expenditure |
| Wang et al., 2012 [73] | 90 subjects with metabolic syndrome | Randomised parallel arm, dietary advice/12 weeks | Pistachios | 42 g/day pistachios plus AHA Step 1 diet vs. 70 g/day pistachio plus AHA Step 1 diet vs. AHA step 1 diet | ↔ body weight,↔ fasting glucose, ↔ 2-h postprandial glucose among treatments |
| ↓ 2-h postprandial glucose within the pistachio groups | |||||
| ↔ TC, ↔ TG (p = 0.018), ↔ LDL-C, ↔HDL-C | |||||
| Holligan et al., 2014 [74] | 28 subjects with elevated LDL levels | Randomised, cross-over, controlled-/4 weeks | Pistachios | 20% energy of a diet replaced with 63–126 g/day of pistachios vs. 10% energy of a diet replaced with 32–63 g/day of pistachios vs. low-fat control diet | ↔ body weight, ↔ energy intake |
| ↓ small, dense LDL-C levels compared with the low-fat control diet. | |||||
| ↓ triacylglycerol/HDL-C on 63–126 g/day of pistachio diet compared with the control diet | |||||
| Razquin et al., 2017 [78] | 4242 subjects | Randomised controlled/3 years | Walnuts, Almonds, Hazelnuts | Mediterranean diets enriched with 30 g/day of nuts (15 g/day walnuts, 7.5 g/day hazelnuts, and 7.5 g/day almonds) vs. Mediterranean diets enriched with 50 mL/day of extra virgin olive oil vs. low-fat diet | ↓ body weight |
| (↓ ~5 kg in the two lowest quintiles of energy density | |||||
| ↓ ~4 kg the highest quintile of energy density | |||||
| ↓ ~2 kg in the two middle quintiles of energy density) |
| Reference | Subjects | Study Design/Period | Nut Type | Diet Intervention | Results |
|---|---|---|---|---|---|
| Durak et al., 1999 [77] | 30 healthy subjects | Pre-post supplemental, dietary advice | Hazelnuts | 1 g/kg body weight/day of hazelnuts supplemented with a habitual diet vs. baseline | ↑ 0.5 kg body weight |
| ↓ TC (p < 0.005) , ↓ LDL-C (p < 0.0005), ↑ HDL-C, ↑ TG (p< 0.001), ↑ HDL-C/LDL-C, (p < 0.0005) | |||||
| ↓ MDA(p < 0.0005), ↑ AOP (p < 0.0005) after hazelnut supplementation | |||||
| Kocyigit et al., 2006 [71] | 44 health subjects | Randomised parallel arm/3 weeks | Pistachios | 20% energy of regular diet replaced with pistachio vs. regular diet | ↔ body weight, ↔ energy intake |
| ↓ TC, ↓ MDA, ↓ TC/HDL-C (p < 0.001) | |||||
| ↓ LDL-C/HDL-C (p < 0.01), ↑ HDL-C (p < 0.001), ↑ AOP, ↑ AOP/MDA | |||||
| Jia et al., 2006 [205] | 30 healthy adult male regular smokers | Parallel arm, control/4 weeks | Almonds | 84 g/day almond group (n = 10) vs. 168 g/day almond group (n = 10) vs. Control group without nuts (n = 10) | ↓ urinary 8-OH-dG, ↓ single strand DNA breaks, |
| ↓MDA, ↔ SOD, ↔ GSH-Px in almond groups compared with the control group | |||||
| Haddad et al., 2006 [212] | 24 healthy subjects | Randomized, controlled, crossover/4 weeks | Pecans | 20% energy of a diet replaced with pecans vs. a control diet without nuts | ↓ MDA, ↔ferric-reducing ability, ↔Trolox equivalent antioxidant capacity |
| Davis et al., 2007 [206] | 65 subjects with metabolic syndrome | Randomised, parallel arm, control/8 weeks | Walnuts | 20% energy of a habitual diet replaced with walnuts (63–108 g/day) vs. 20% energy of a habitual diet replaced with cashews (63–108 g/day) vs. Control diet without nuts | ↓ GSSG, ↑ ORAC compared with baseline |
| Garg et al., 2007 [207] | 17 male hypercholesterolemic subjects | Pre-post supplemental/4 weeks | Macadamias | 15% energy of a habitual diet replaced with macadamias (40–90 g/day) vs. baseline | ↓ oxidative status (8-isoprostane) |
| ↓ inflammation (leukotriene, LTB4) | |||||
| ↔ TXB2/PGI2 ratio | |||||
| Li et al., 2007 [208] | 60 healthy male habitual smokers | Randomized, crossover/4 weeks | Almonds | Smokers; 84 g/day almonds vs. 120 g/day of pork (for smokers) | ↑ serum a-tocopherol, ↑ SOD, ↑ GPX, |
| 30 healthy male | ↔ caltalase after almond intake compared with no change in smokers after pork intake | ||||
| non-smokers | Non-smokers; 120 g/day of pork for reference comparison | ↓ DNA strand breaks, ↓ 8-OHdG, ↓ MDA, in smokers after almond intake compared with baseline | |||
| Jenkins et al., 2008 [209] | 27 hyperlipidemic subjects‘ | Randomized, crossover/4 weeks | Almonds | 73 g/day almonds added to self-selected low-fat therapeutic diet vs. 36.5 g/day almonds added to self-selected low-fat therapeutic diet vs. self-selected low-fat therapeutic diet without nuts (control) | ↓ MDA, ↓ urinary isoprostane on 73 g/day almonds compared with control |
| ↔ α-or γ-tocopherol, | |||||
| Thomson et al., 2008 [203] | 59 healthy subjects | Randomized controlled/12 weeks | Brazil nuts | 2 Brazil nuts (providing ≈100 μg Se, 100 μg Se as selenomethionine) vs. Placebo | ↑ selenium (p < 0.0001), ↑ GPx (p < 0.001) |
| McKay et al., 2010 [213] | 21 non-smoking man and postmenopausal women aged over 50 years | Randomized crossover/6 weeks | Walnuts | 42 g/day walnuts vs. 21 g/day walnuts | ↔ antioxidant activity (ORAC, ORAC with perchloric acid (pca) precipitation, FRAP and TAP), ↔ biomarkers of antioxidant status (total thiols, phenols, carotenoids and GPx), ↔ MDA |
| Lopez-Uriarte et al., 2010 [121] | 50 subjects with metabolic syndrome | Randomised, controlled, parallel/4 weeks | Mixed nuts | 30 g/day mix nuts (15 g walnuts, 7.5 g almonds and 7.5 g hazelnuts) vs. control diet without nuts | ↓ DNA damage (p < 0.001), |
| ↔ Antioxidant capacity, ↔oxidized LDL, ↔ conjugated diene, | |||||
| ↔ 8-iso-prostanes, ↔ endothelial function | |||||
| Kay et al., 2010 [210] | 28 hypercholesterolemic subjects | Randomized, crossover controlled/4 weeks | Pistachios | 20% energy of a low-fat diet replaced with 63–126 g/day pistachios vs. 20% energy of a low-fat diet replaced with 32–63 g/day pistachios vs. a low-fat control diet without pistachios | ↓ oxidized-LDL compared with a low-fat control diet |
| ↑ lutein (p < 0.0001), ↑ α-carotene, ↑ β-carotene (p < 0.01)compared with baseline | |||||
| Sari et al., 2010 [24] | 32 healthy young men | Dietary intervention/8 weeks | Pistachios | Subjects consumed a Mediterranean diet for 4 weeks and in the next 4 weeks they supplemented the Mediterranean diet with pistachios for 4 weeks replacing 32% of the energy obtained from MUFAs in the control diet (20% of energy) | ↓ glucose levels (−8.8 ± 8.5% p < 0.001), |
| ↓ TC ( p < 0.001) ↓ LDL-C (p < 0.001), ↓ triacylglycerol, ↔ HDL-C, ↓ TC-C/HDL-C ↓ LDL-C/HDL-C ( p < 0.001), | |||||
| ↑ endothelium-dependent vasodilation (p = 0.002) | |||||
| ↓ lipid hydroperoxide (p < 0.001), | |||||
| ↓ MDA (p < 0.001), ↓ IL-6 (p < 0.001), | |||||
| ↑ SOD (p < 0.001), ↔ hs CRP, ↔ TNF-α | |||||
| compared with a Mediterranean diet | |||||
| Haddad et al., 2014 [211] | healthy young subjects. | Randomized, crossover, controlled, acute 5-h test | Walnuts | A walnut meal containing 90 g walnuts and a isocaloric control meal (50% carbohydrate; 20% protein; 30% fat) | ↓ oxidative stress, ↑ iAUC for hydrophilic and lipophilic ORAC, ↓ iAUC for MDA |
| ↓ oxidized LDL, ↑ epicatechin gallate | |||||
| ↑ epicallocatechin gallate, ↑ urolithin-A in urine |
| Reference | Subjects | Study Design/Period | Nut Type | Diet Intervention | Results |
|---|---|---|---|---|---|
| Zhao et al., 2004 & 2007 [222,223] | 23 hypercholesterolemic subjects | Randomized, controlled cross-over/6 weeks | Walnuts | Three diets; - AAD—13% energy from SFA, 13% energy from MUFAs and 8.7% energy from PUFAs (7.7% LA; 0.8% ALA) - The LA diet consisted of 16.4% energy from PUFAs (12.6% LA; 3.6% ALA) - The ALA diet consisted of 17% energy from PUFAs (10.5% LA; 6.5% ALA). Walnuts and walnut oil were used for half of total fat in the LA and ALA diets. The ratios of LA (n-6) to ALA (n-3) for the AAD, LA and ALA diet were 10:1, 4:1, and 2:1 | ↓ hs-CRP (p < 0.01) on ALA diet compared with the AAD |
| ↓ IL-6, ↓ IL-1β, ↓ TNF-α cultured in PBMCs on the ALA diet than on the LA diet | |||||
| ↓ TC, ↓ TG, ↓ LDL-C ↓ ICAM-1 on both ALA and LA diets compared with AAD diet | |||||
| ↓ VCAM-1 (p < 0.01), ↓ E-selectin (p < 0.01) on the ALA diet than on the LA diet | |||||
| ↓ HDL-C and apolipoprotein AI on ALA diet compared with AAD diet | |||||
| Garg et al., 2007 [207] | 17 male hypercholesterolemic subjects | Pre-post supplemental/4 weeks | Macadamias | 15% energy of a habitual diet replaced with macadamias (40–90 g/day) vs. baseline | ↓ oxidative status (8-isoprostane) |
| ↓ inflammation (leukotriene, LTB4) | |||||
| ↔ TXB2/PGI2 ratio | |||||
| Rajaram et al., 2010 [224] | 25 health subjects | Randomised, controlled, crossover/4 weeks | Almonds | High-almond diet (20% energy of a control diet replaced with almonds) vs. Low-almond diet (10% energy of a control diet replaced with almonds) vs. Heart-healthy control diet without nuts | ↓ hs -CRP ↓ E-selectin (p < 0.0001) |
| Sari et al., 2010 [24] | 32 healthy young men | Dietary intervention/8 weeks | Pistachios | Subjects consumed a Mediterranean diet for 4 weeks and in the next 4 weeks they supplemented the Mediterranean diet with pistachios for 4 weeks replacing 32% of the energy obtained from MUFAs in the control diet (20% of energy) | ↓ glucose levels (−8.8 ± 8.5% p < 0.001) |
| ↓ TC, ↓ LDL-C, ↓ triacylglycerol, ↔ HDL-C, ↓ TC-C/HDL-C ↓ LDL-C/HDL-C | |||||
| ↑ endothelium-dependent vasodilation | |||||
| ↓ lipid hydroperoxide, ↓ MDA, ↓ IL-6, | |||||
| ↑ SOD, ↔ hs CRP, ↔ TNF-α | |||||
| compared with a Mediterranean diet | |||||
| Hernandez-Alonso et al., 2014 [225] | 54 subjects with prediabetes | Randomized, controlled cross-over/4 months | Pistachios | A diet supplemented with 57 g/day pistachio vs. a control diet | ↓ IL-6 mRNA, ↓ resistin gene expression |
| ↑ SLC2A4 expression | |||||
| ↓ fasting glucose, ↓ insulin, ↓ HOMA-IR | |||||
| ↓ fibrinogen, ↓ oxidized LDL, ↓ platelet factor 4 | |||||
| ↑ GLP-1 | |||||
| Sauder et al., 2015 [22] | 30 subjects with well-controlled T2DM | Randomized, crossover, controlled/4 weeks | Pistachios | Healthy diet with pistachios contributing 20% of total energy. Low-fat or fat-free snacks (e.g., pretzels) in the control diet were substituted with roasted pistachios providing 20% of daily energy (59 to 128 g/day of pistachios). Half of amount of pistachio was ingested unsalted vs. AHA Therapeutic Lifestyle Changes diet (26.9% total fat, 6.7% SFA, 186 mg/day cholesterol). | ↓ fructosamine, ↓ TC, ↓ ratio of TC to HDL-C, ↓ TG (p = 0.003), ↔ HbA1c, |
| ↔ fasting glucose, ↔ insulin, ↔ hs-CRP, | |||||
| ↔ ICAM, ↔ VCAM, ↔endothelia function | |||||
| Arpon et al., 2017 [221] | 36 subjects at high cardiovascular risks | Randomised, controlled, parallel/5 years | Mixed nuts | Mediterranean diet supplemented with nuts (30 g/day of nuts) vs. Mediterranean diet supplemented with extra virgin olive oil (1 L/week of virgin olive oil) vs. Low-fat diet (control) | ↑ association between methylation changes of 8 genes (EEF2, COL18A1, IL4I1, LEPR, PLAGL1, IFRD1, MAPKAPK2, and PPARGC1B) related to inflammation and Mediterranean diets ↑ association between EEF2 methylation and TNF-α and hs-CRP on Mediterranean diets compared with baselines. |
| Gulati et al., 2017 [226] | 52 subjects with T2DM | Pre-post supplemental study, dietary advice, physical activity (45 min of walking at least 5 days some week)/24 weeks | Almonds | 20% of total energy of a diet replaced with almonds vs. baseline | ↓ hs-CRP (p < 0.01), |
| ↓ waist circumference, | |||||
| ↓ waist-to-height ratio, | |||||
| ↓ TC, ↓ TG, ↓ LDL-C, ↓ HbA1c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients 2017, 9, 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9111271
Kim Y, Keogh JB, Clifton PM. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients. 2017; 9(11):1271. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9111271
Chicago/Turabian StyleKim, Yoona, Jennifer B. Keogh, and Peter M. Clifton. 2017. "Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions" Nutrients 9, no. 11: 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9111271