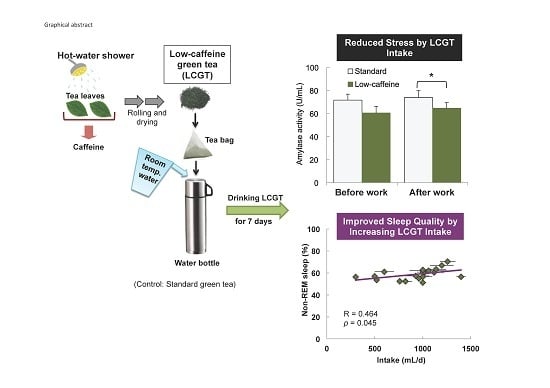
Reduced Stress and Improved Sleep Quality Caused by Green Tea Are Associated with a Reduced Caffeine Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LCGT
2.2. Measurement of Tea Components by HPLC
2.3. Participants
2.4. Procedure
2.5. Measurement of sAA
2.6. Measurement of EEG
2.7. Statistical Analysis
3. Results
3.1. Tea Components in SGT and LCGT
3.2. Interaction between sAA Level and LCGT Intake
3.3. Effect of SGT and LCGT Ingestion on Sleep Parameters
3.4. Subjective Stress and Fatigue
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grove, K.A.; Lambert, J.D. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J. Nutr. 2010, 140, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef] [PubMed]
- Horie, H.; Kohata, K. General ingredients of tea. In Health Science of Tea, New Possibility for Physiological Function; Muramatsu, K., Oguni, I., Isemura, M., Sugiyama, K., Yamamoto-Maeda, M., Eds.; Gakkai Shuppan Center: Tokyo, Japan, 2002; pp. 26–31. [Google Scholar]
- Unno, K.; Fujitani, K.; Takamori, N.; Takabayashi, F.; Maeda, K.; Miyazaki, H.; Tanida, N.; Iguchi, K.; Shimoi, K.; Hoshino, M. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 2011, 45, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Iguchi, K.; Tanida, N.; Fujitani, K.; Takamori, N.; Yamamoto, H.; Ishii, N.; Nagano, H.; Nagashima, T.; Hara, A.; et al. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp. Physiol. 2013, 98, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Tanida, N.; Ishii, N.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; Takeda, A.; Ozawa, H.; Ohkubo, T.; Juneja, L.R.; et al. Anti-stress effect of theanine on students during pharmacy practice: Positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013, 111, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol. Res. 2011, 64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Miyazaki, Y.; Unno, K.; Min, J.Z.; Todoroki, K.; Toyo’oka, T. Stable isotope dilution HILIC-MS/MS method for accurate quantification of glutamic acid, glutamine, pyroglutamic acid, GABA and theanine in mouse brain tissues. Biomed. Chromatogr. 2016, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Prabhakar, M.; Kumar, P.; Deshmukh, R.; Sharma, P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013, 698, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine. Phytomedicine 2015, 23, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Yamada, H.; Iguchi, K.; Ishida, H.; Iwao, Y.; Morita, A.; Nakamura, Y. Anti-stress effect of green tea with lowered caffeine on human: A pilot study. Biol. Pharm. Bull. 2017, 40, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Shimotoku, T.; Ichikawa, H.; Anan, T.; Takayanagi, H.; Ikegaya, K. Relation between amounts of some ingredients extracted from green tea and brewing conditions. Tea Res. J. (Chagyo Kenkyu Hokoku) 1982, 55, 43–50. [Google Scholar] [CrossRef]
- Monobe, M.; Ema, K.; Tokuda, Y.; Maeda-Yamamoto, M. Effect on the epigallocatechin gallate/epigallocatechin ratio in a green tea (Camellia sinensis L.) extract of different extraction temperatures and its effect on IgA production in mice. Biosci. Biotechnol. Biochem. 2010, 74, 2501–2503. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Almela, M.; Hidalgo, V.; Villada, C.; van der Meij, L.; Espín, L.; Gómez-Amor, J. Salvador A. Salivary alpha-amylase response to acute psychosocial stress: The impact of age. Biol. Psychol. 2011, 87, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; La Marca, R.; Florin, L.; Moses, A.; Langhans, W.; Koller, M.M.; Ehlert, U. Stress-induced changes in human salivary alpha-amylase activity–associations with adrenergic activity. Psychoneuroendocrinology 2006, 31, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.C.; Bennett, J.M.; Whetzel, C.A.; Granger, D.A.; Ritter, F.E. Caffeine and stress alter salivary alpha-amylase activity in young men. Hum. Psychopharmacol. 2010, 25, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; Shrager, S.E.; Shea, S. Association of sleep duration and quality with alterations in the hypothalamic-pituitary adrenocortical axis: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Endocrinol. Metab. 2015, 100, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Räikkönen, K.; Matthews, K.A.; Pesonen, A.K.; Pyhälä, R.; Paavonen, E.J.; Feldt, K.; Jones, A.; Phillips, D.I.; Seckl, J.R.; Heinonen, K.; et al. Poor sleep and altered hypothalamic-pituitary- adrenocortical and sympatho-adrenal-medullary system activity in children. J. Clin. Endocrinol. Metab. 2010, 95, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Horie, H.; Maeda-Yamamoto, M.; Ujihara, T.; Kohata, K. Extraction of tea catechins for chemical analysis. Tea Res. J. (Chagyo Kenkyu Hokoku) 2002, 94, 60–64. [Google Scholar] [CrossRef]
- Goto, T.; Horie, H.; Mukai, T. Analysis of major amino acids in green tea by high-performance liquid chromatography coupled with OPA precolumn derivatization. Tea Res. J. (Chagyo Kenkyu Hokoku) 1993, 77, 29–33. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Takai, N.; Mizuno, Y.; Yoshida, H. Performance evaluation of salivary amylase activity monitor. Biosens. Bioelectron. 2004, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kashiwagi, K.; Kadotani, H.; Yamamoto, K.; Koike, S.; Matsuo, M.; Yamada, N.; Okawa, M.; Urade, Y. Validation of a portable single-channel EEG monitoring system. J. Oral Sleep Med. 2015, 1, 140–147. [Google Scholar]
- Yoshida, M.; Shinohara, H.; Kodama, H. Assessment of nocturnal sleep architecture by actigraphy and one-channel electroencephalography in early infancy. Early Hum. Dev. 2015, 91, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Flexer, A.; Gruber, G.; Dorffner, G. A reliable probabilistic sleep stager based on a single EEG signal. Artif. Intell. Med. 2005, 33, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Ingestion of green tea with lowered caffeine improves sleep quality of the elderly via suppression of stress. J. Clin. Biol. Nutr. 2017, 61, 1–7. [Google Scholar]
- Lui, S.L.; Ng, F.; Lo, W.K. Factors associated with sleep disorders in Chinese patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2002, 22, 677–682. [Google Scholar] [PubMed]
- Janson, C.; Gislason, T.; De Backer, W.; Plaschke, P.; Björnsson, E.; Hetta, J.; Kristbjarnason, H.; Vermeire, P.; Boman, G. Prevalence of sleep disturbances among young adults in three European countries. Sleep 1995, 18, 589–597. [Google Scholar] [PubMed]
- Kobayashi, H.; Demura, S.; Goshi, F.; Minami, M.; Nagasawa, Y.; Sato, S. Trends in variation within the day and between days of subjective symptoms of fatigue in adolescent males. Nihon Eiseigaku Zasshi 2000, 54, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Takahashi, M.; Sato, T.; Sasaki, T.; Oka, T.; Iwasaki, K. Weekend sleep intervention for workers with habitually short sleep periods. Scand. J. Work Environ. Health 2011, 37, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hartzler, B.M. Fatigue on the flight deck: The consequences of sleep loss and the benefits of napping. Accid. Anal. Prev. 2014, 62, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Woodson, P.P. Caffeine physical dependence: A review of human and laboratory animal studies. Psychopharmacology (Berl.) 1988, 94, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; Mcleland, J.S.; Toedebusch, C.D.; Boyd, J.; Morris, J.C.; Landsness, E.C.; Yamada, K.; Holtzman, D.M. Comparison of a single-channel EEG sleep study to polysomnography. J. Sleep Res. 2016, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]





| Term (1 Term with 7 Days) | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Green tea intake | Washout (water) | SGT or LCGT | Washout (water) | LCGT or SGT |
| (Saturday~Friday) | (Saturday~Friday) | (Saturday~Friday) | (Saturday~Friday) | |
| Measurement of α-amylase activity | (-) | 2 times/day (morning and evening) | (-) | 2 times/day (morning and evening) |
| Subjective stress and Physical condition | (-) | every evening | (-) | every evening |
| Sleep | (-) | 3 nights/week (Tuesday~Thursday) | (-) | 3 nights/week (Tuesday~Thursday) |
| Subjective fatigue | (-) | 2 times/week (Mon morning and Fri evening) | (-) | 2 times/week (Monday morning and Friday evening) |
| Sleep Parameters | Abbreviation | Standard Green Tea | Low-Caffeine Green Tea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Correlation between sAA before Work | Correlation between sAA after Work | Correlation between Intake Volume | Mean ± SEM | Correlation between sAA before Work | Correlation between sAA after Work | Correlation between Intake Volume | ||||||||
| R | p Value | R | p Value | R | p Value | R | p Value | R | p Value | R | p Value | ||||
| Onset of sleep (min) | SL | 12.6 ± 3.0 | −0.129 | 0.599 | −0.239 | 0.325 | 0.356 | 0.135 | 15.7 ± 4.9 | 0.075 | 0.759 | −0.026 | 0.915 | −0.138 | 0.572 |
| Onset of REM (min) | REM SL | 63.5 ± 5.3 | 0.184 | 0.450 | −0.199 | 0.415 | 0.427 | 0.068 | 57.7 ± 3.0 | 0.045 | 0.854 | −0.045 | 0.854 | 0.304 | 0.207 |
| Total period of sleep (min) | SPT | 351.3 ± 12.5 | 0.462 | 0.046 * | 0.232 | 0.339 | −0.136 | 0.579 | 350.2 ± 14.6 | 0.173 | 0.479 | 0.024 | 0.921 | 0.301 | 0.211 |
| Total sleep time (SPT−WASO) (min) | TST | 328.7 ± 12.7 | 0.484 | 0.036 * | 0.2672 | 0.269 | −0.138 | 0.572 | 328.6 ± 13.5 | 0.130 | 0.595 | 0.040 | 0.872 | 0.309 | 0.198 |
| Rapid eye movement (%) | REM | 28.2 ± 2.5 | 0.066 | 0.787 | −0.082 | 0.739 | −0.106 | 0.665 | 25.9 ± 1.2 | 0.077 | 0.753 | 0.142 | 0.562 | −0.186 | 0.446 |
| Light sleep (%) | N1 | 8.7 ± 0.7 | 0.361 | 0.129 | 0.2039 | 0.402 | −0.055 | 0.822 | 9.3 ± 0.8 | 0.285 | 0.236 | 0.375 | 0.113 | −0.438 | 0.061 |
| Slow wave sleep (%) | N2+N3 | 58.8 ± 1.4 | 0.197 | 0.420 | 0.1261 | 0.607 | −0.137 | 0.577 | 58.7 ± 1.2 | −0.411 | 0.080 | −0.351 | 0.141 | 0.464 | 0.045 * |
| Time spent awake during the sleep (min) | WASO | 22.6 ± 2.9 | −0.117 | 0.635 | −0.163 | 0.505 | 0.018 | 0.943 | 21.7 ± 2.3 | 0.329 | 0.169 | −0.076 | 0.757 | 0.096 | 0.697 |
| Total awakening time during two hours before the final awakening (min) | B2 WASO | 18.5 ± 4.4 | 0.009 | 0.969 | −0.219 | 0.367 | −0.126 | 0.607 | 9.7 ± 1.5 | −0.018 | 0.941 | −0.139 | 0.571 | 0.250 | 0.301 |
| Average sleep cycle (min) | AVR SLC | 91.5 ± 3.2 | −0.032 | 0.895 | −0.268 | 0.267 | −0.189 | 0.437 | 90.4 ± 2.9 | 0.006 | 0.979 | −0.248 | 0.306 | 0.176 | 0.471 |
| Sleep eficiency (%) | SE | 88.5 ± 1.7 | 0.099 | 0.688 | 0.281 | 0.244 | −0.122 | 0.620 | 89.2 ± 1.5 | −0.153 | 0.533 | 0.055 | 0.824 | 0.110 | 0.654 |
| δ power in the 1st sleep cycle (µV2) | 1st δ | 149,256 ± 18,656 | −0.031 | 0.900 | −0.157 | 0.520 | −0.052 | 0.832 | 120644 ± 15923 | −0.019 | 0.938 | 0.158 | 0.518 | −0.254 | 0.295 |
| sAA (U/mL) | 71.7 ± 5.2 | 73.9 ± 5.9 | 60.5 ± 4.3 | 64.7 ± 4.8 | |||||||||||
| Intake volume (mL) | 929 ± 31 | 927 ± 34 | |||||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced Stress and Improved Sleep Quality Caused by Green Tea Are Associated with a Reduced Caffeine Content. Nutrients 2017, 9, 777. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9070777
Unno K, Noda S, Kawasaki Y, Yamada H, Morita A, Iguchi K, Nakamura Y. Reduced Stress and Improved Sleep Quality Caused by Green Tea Are Associated with a Reduced Caffeine Content. Nutrients. 2017; 9(7):777. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9070777
Chicago/Turabian StyleUnno, Keiko, Shigenori Noda, Yohei Kawasaki, Hiroshi Yamada, Akio Morita, Kazuaki Iguchi, and Yoriyuki Nakamura. 2017. "Reduced Stress and Improved Sleep Quality Caused by Green Tea Are Associated with a Reduced Caffeine Content" Nutrients 9, no. 7: 777. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9070777