Chlorotoxin—A Multimodal Imaging Platform for Targeting Glioma Tumors
Abstract
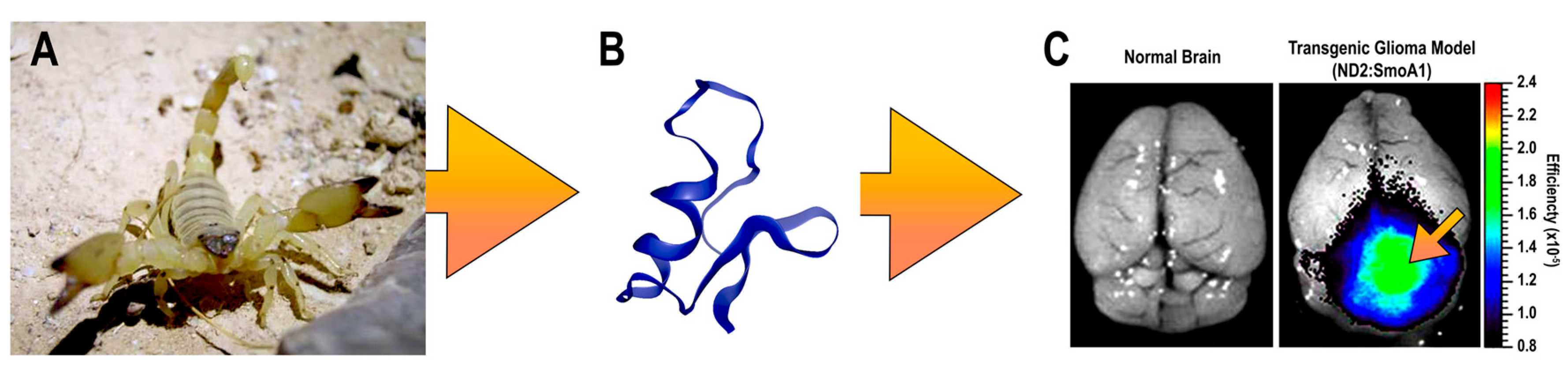
:1. Chlorotoxin (CTX) as A Potential Targeting Agent for Glioblastoma
2. CTX Structure and Molecular Targets
3. Molecular Imaging of Glioblastoma
3.1. CTX-Based Nuclear Imaging
3.2. CTX-Based MRI/Optical Imaging
3.3. US/Optoacoustic Imaging of Glioma: Current Progress and Expectations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015, 17, iv1–iv62. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Ammirati, M.; Bierman, P.J.; Brem, H.; Butowski, N.; Chamberlain, M.C.; DeAngelis, L.M.; Fenstermaker, R.a.; Friedman, A.; Gilbert, M.R.; Hesser, D.; et al. NCCN clinical practice guidelines in oncology: Central nervous system cancers. J. Natl. Compr. Cancer Netw. 2014, 15, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5. [Google Scholar] [CrossRef]
- Mukherjee, D.; Quinones-Hinojosa, A. Impact of Extent of Resection on Outcomes in Patients with High-Grade Gliomas; Hayat, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-0618-7. [Google Scholar]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Shi, C.; Liu, Y.; Teng, L.; Han, D.; et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-Aminolevulinic scid-induced porphyrins: A dystematic review and meta-analysis of prospective studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitucci, M.; Hayes, D.N.; Miller, C.R. Gene expression profiling of gliomas: Merging genomic and histopathological classification for personalised therapy. Br. J. Cancer 2011, 104, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar] [PubMed]
- Olsen, M.L.; Schade, S.; Lyons, S.A.; Amaral, M.D.; Sontheimer, H. Expression of voltage-gated chloride channels in human glioma cells. J. Neurosci. 2003, 23, 5572–5582. [Google Scholar] [CrossRef] [PubMed]
- McFerrin, M.B.; Sontheimer, H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; He, B.; Dai, W.; Lin, Z.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Wang, G.; Yin, L.; et al. The impact of a chlorotoxin-modified liposome system on receptor MMP-2 and the receptor-associated protein ClC-3. Biomaterials 2014, 35, 5908–5920. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Biopolymers 2016, 106, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippens, G.; Najib, J.; Tartar, A.; Lippens, G.; Wodak, S.J. NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry 1995, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Akcan, M.; Stroud, M.R.; Hansen, S.J.; Clark, R.J.; Daly, N.L.; Craik, D.J.; Olson, J.M. Chemical re-engineering of chlorotoxin improves bioconjugation properties for tumor imaging and targeted therapy. J. Med. Chem. 2011, 54, 782–787. [Google Scholar] [CrossRef] [PubMed]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef] [PubMed]
- Bontems, F.; Gilquin, B.; Roumestand, C.; Ménez, A.; Toma, F. Analysis of side-chain organization on a refined model of charybdotoxin: Structural and functional implications. Biochemistry 1992, 31, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, N.; Bordey, A.; Gillespie, G.Y.; Sontheimer, H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience 1998, 83, 1161–1173. [Google Scholar] [CrossRef]
- Ullrich, N.; Sontheimer, H. Cell cycle-dependent expression of a glioma-specific chloride current: Proposed link to cytoskeletal changes. Am. J. Physiol. 1997, 273, C1290–C1297. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Sontheimer, H. Cl− and K+ channels and their role in primary brain tumour biology. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130095. [Google Scholar] [CrossRef] [PubMed]
- Maertens, C.; Wei, L.; Tytgat, J.; Droogmans, G.; Nilius, B. Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels. Br. J. Pharmacol. 2000, 129, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Ween, M.P.; Oehler, M.K.; Ricciardelli, C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N. Targeted antitumor therapy with the scorpion venom chlorotoxin. Drugs Future 2011, 36, 615. [Google Scholar] [CrossRef]
- Kesavan, K.; Ratliff, J.; Johnson, E.W.; Dahlberg, W.; Asara, J.M.; Misra, P.; Frangioni, J.V.; Jacoby, D.B. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J. Biol. Chem. 2010, 285, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Tatenhorst, L.; Rescher, U.; Gerke, V.; Paulus, W. Knockdown of annexin 2 decreases migration of human glioma cells in vitro. Neuropathol. Appl. Neurobiol. 2006, 32, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-based peptide therapy: Insights into anti-cancer mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Puttick, S.; Bell, C.; Dowson, N.; Rose, S.; Fay, M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov. Today 2015, 20, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, L.; Rani, D.; El Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; An, N.; Li, K.; Zheng, Y.; Liang, A. Chlorotoxin-conjugated nanoparticles as potential glioma-targeted drugs. J. Neurooncol. 2012, 107, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Khazaeli, M.B.; Gillespie, G.Y.; Alvarez, V.L. Radiation dosimetry of 131I-chlorotoxin for targeted radiotherapy in glioma-bearing mice. J. Neurooncol. 2005, 71, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, D.C.; Shen, S.; Fiveash, J.; Raubitschek, A.; Colcher, D.; Liu, A.; Alvarez, V.; Mamelak, A.N. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 2005, 46, 580–586. [Google Scholar] [PubMed]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Shi, X.; Zhao, J. Chlorotoxin-conjugated multifunctional dendrimers labeled with radionuclide 131I for single photon emission computed tomography imaging and radiotherapy of gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Naddaka, M.; Uboldi, C.; Loudos, G.; Fragogeorgi, E.; Molinari, V.; Pucci, A.; Tsotakos, T.; Psimadas, D.; Ponti, J.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, L.; Qiao, W.; Xing, Y.; Zhao, J. BmK CT and 125I-BmK CT suppress the invasion of glioma cells in vitro via matrix metalloproteinase-2. Mol. Med. Rep. 2017, 15, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Jian, X.C.; Yin, B.; He, Z.J. Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chin. J. Cancer 2010, 29, 626–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiseh, M.; Gabikian, P.; Bahrami, S.B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.L.; Curtis, E.; Othman, S.F.; Simpson, M.A.; Olive, D.M. Characterization of IRDye 800CW chlorotoxin as a targeting agent for brain tumors. Anal. Biochem. 2013, 440, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem. 2011, 50, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.F.; Sun, Z.; Li, M.; Xiang, Y.; Wang, Q.Q.; Tang, F.; Wu, Y.; Cao, Z.; Li, W. Neurotoxin-conjugated upconversion nanoprobes for direct visualization of tumors under near-infrared irradiation. Biomaterials 2010, 31, 8724–8731. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ahmadiantehrani, M.; Publicover, N.G.; Hunter, K.W.J.; Zhu, X. Thermal decomposition based synthesis of Ag-In-S/ZnS quantum dots and their chlorotoxin-modified micelles for brain tumor cell targeting. RSC Adv. 2015, 74, 60612–60620. [Google Scholar] [CrossRef] [PubMed]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-infrared imaging of brain tumors using the tumor paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiseh, O.; Kievit, F.M.; Gunn, J.W.; Ratner, B.D.; Zhang, M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials 2009, 30, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Fang, C.; Stephen, Z.; Veiseh, O.; Hansen, S.; Lee, D.; Ellenbogen, R.G.; Olson, J.; Zhang, M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine 2008, 3, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Meng, X.; Liu, E.; Chen, K. Incorporation of magnetite nanoparticle clusters in fluorescent silica nanoparticles for high-performance brain tumor delineation. Nanotechnology 2010, 21, 235104. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.E.; Veiseh, O.; Bhattarai, N.; Sun, C.; Hansen, S.J.; Ditzler, S.; Knoblaugh, S.; Lee, D.; Ellenbogen, R.; Zhang, M.; et al. Rapid pharmacokinetic and biodistribution studies using cholorotoxin-conjugated iron oxide nanoparticles: A novel non-radioactive method. PLoS ONE 2010, 5, e9536. [Google Scholar] [CrossRef]
- Sun, C.; Veiseh, O.; Gunn, J.; Fang, C.; Hansen, S.; Lee, D.; Sze, R.; Ellenbogen, R.G.; Olson, J.; Zhang, M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 2008, 4, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Sun, C.; Gunn, J.; Kohler, N.; Gabikian, P.; Lee, D.; Bhattarai, N.; Ellenbogen, R.; Sze, R.; Hallahan, A.; et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005, 5, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, H.; Gu, W.; Li, S.; Xiao, N.; Shao, C.; Xu, Q.; Ye, L. Ho3+ doped NaGdF4 nanoparticles as MRI/optical probes for brain glioma imaging. J. Mater. Chem. B 2014, 2, 1521–1529. [Google Scholar] [CrossRef]
- Huang, R.; Han, L.; Li, J.; Liu, S.; Shao, K.; Kuang, Y.; Hu, X.; Wang, X.; Lei, H.; Jiang, C. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials 2011, 32, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, J.; Qiao, W.; Chen, K. Recent advances in diagnosis and treatment of gliomas using chlorotoxin-based bioconjugates. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 385–405. [Google Scholar] [PubMed]
- Lebduková, P.; Sour, A.; Helm, L.; Tóth, É.; Kotek, J.; Luke, I.; Merbach, A.E. Phosphinic derivative of DTPA conjugated to a G5 PAMAM dendrimer: An17O and1H relaxation study of its Gd(III) complex. Dalt. Trans. 2006, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, P.; Nesbit, G.; Muldoon, L.L.; Nixon, R.R.; Delashaw, J.; Cohen, J.I.; Petrillo, A.; Rink, D.; Neuwelt, E.A. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. Am. J. Neuroradiol. 2002, 23, 509–510. [Google Scholar]
- Momtazi, L.; Bagherifam, S.; Singh, G.; Hofgaard, A.; Hakkarainen, M.; Glomm, W.R.; Roos, N.; Maelandsmo, G.M.; Griffiths, G.; Nystrom, B.; et al. Synthesis, characterization, and cellular uptake of magnetic nanocarriers for cancer drug delivery. J. Colloid Interface Sci. 2014, 1, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.S.; Zhang, J.L.; Liu, G.; Zhang, W.G. Application of iron oxide nanoparticles in glioma imaging and therapy: From bench to bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.V.; Moore, A.; Medarova, Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm. Res. 2012, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jun, Y.W.; Yeon, S.I.; Kim, H.C.; Shin, J.S.; Cheon, J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, X.; Zhao, J. Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma. Curr. Top. Med. Chem. 2015, 15, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.H.; Zhang, M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small 2009, 5, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Staderini, M.; Megia-Fernandez, A.; Dhaliwal, K.; Bradley, M. Peptides for optical medical imaging and steps towards therapy. Bioorg. Med. Chem. 2018, 26, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Michalet, X.; Chang, Y.P.; Pinaud, F.F.; Matyas, S.E.; Payne, G.; Weiss, S. High affinity scFv-hapten pair as a tool for quantum dot labeling and tracking of single proteins in live cells. Nano Lett. 2008, 8, 4618–4623. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L. Quantum dots, lighting up the research and development of nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 385–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauck, T.S.; Anderson, R.E.; Fischer, H.C.; Newbigging, S.; Chan, W.C.W. In vivo quantum-dot toxicity assessment. Small 2010, 6, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.S.; Kele, P.; Saleh, S.M.; Wolfbeis, O.S. Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging. Curr. Opin. Chem. Biol. 2010, 14, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Stroud, M.R.; Hansen, S.J.; Olson, J.M. In vivo bio-imaging using chlorotoxin-based conjugates. Curr. Pharm. Des. 2011, 17, 4362–4371. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Bost, W.; Fournelle, M. Molecular imaging of glioblastoma cells using functionalized nanorods and a high resolution optoacoustic microscope. IEEE Int. Ultrason. Symp. IUS 2013, 120–123. [Google Scholar] [CrossRef]
- Kim, C.; Qin, R.; Xu, J.S.; Wang, L.V.; Xu, R. Multifunctional microbubbles and nanobubbles for photoacoustic and ultrasound imaging. J. Biomed. Opt. 2010, 15, 10510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goertz, D.E. An overview of the influence of therapeutic ultrasound exposures on the vasculature: High intensity ultrasound and microbubble-mediated bioeffects. Int. J. Hyperth. 2015, 31, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.L. The application of microbubbles for targeted drug delivery. Expert Opin. Drug Deliv. 2007, 4, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Lum, A.F.H.; Borden, M.A.; Dayton, P.A.; Kruse, D.E.; Simon, S.I.; Ferrara, K.W. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J. Control. Release 2006, 111, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Shohet, R.V.; Bekeredjian, R.; Frenkel, P.; Grayburn, P.A. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J. Am. Coll. Cardiol. 2003, 42, 301–308. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.I.; Burks, S.R.; Frank, J.A. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 2018, 8, 2245–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Teng, M.C.; Lu, M.; Liang, H.F.; Lee, Y.R.; Yen, C.C.; Liang, M.L.; Wong, T.T. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int. J. Nanomed. 2012, 7, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Fan, C.H.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wang, H.E.; Lin, G.L.; Lin, H.H.; Wong, T.T. Evaluation of the increase in permeability of the blood-brain barrier during tumor progression after pulsed focused ultrasound. Int. J. Nanomed. 2012, 7, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Vykhodtseva, N.; McDannold, N.; Hynynen, K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 2008, 48, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Chen, C.-C.; Kao, Y.-H.; Chen, C.-L.; Ko, C.-E.; Horng, S.-C.; Chen, R.-C. Evaluation of dose distribution of molecular delivery after blood-brain barrier disruption by focused ultrasound with treatment planning. Ultrasound Med. Biol. 2013, 39, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Ko, C.E.; Huang, S.Y.; Chung, I.F.; Chen, G.S. Pharmacokinetic changes induced by focused ultrasound in glioma-bearing rats as measured by dynamic contrast-enhanced MRI. PLoS ONE 2014, 9, e92910. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liang, L.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J. Control. Release 2011, 152, 402–410. [Google Scholar] [CrossRef] [PubMed]
| CTX-Conjugate | Imaging Modality | References |
|---|---|---|
| 131I/125I-CTX (131I-TM601) | SPECT Imaging | [10,35,36,37,38] |
| 131I-BmK-CT | SPECT imaging | [39] |
| Ag/Ali-PNPs-CTX-99mTc | SPECT imaging | [40] |
| 131I-HPAO-PEG-dendrimers-CTX | SPECT imaging | [41,42] |
| Cy5.5-CTX | Optical Imaging | [43] |
| Mono-labeled CTX-Cy5.5 | Optical Imaging | [18] |
| IRDye 800CW-CTX | Optical Imaging | [44] |
| Pdot-CTX | Optical Imaging | [45] |
| PEI-NaYF(4):Yb, Er/Ce-CTX | Optical Imaging | [46] |
| QD(Ag-In-S/ZnS)-CTX | Optical Imaging | [47] |
| ICG-CTX (BLZ100) | Optical Imaging | [48] |
| NP–AF647-(DNA or siRNA)-CTX | Optical Imaging | [49,50] |
| IONP-PEG-CTX | MRI | [51] |
| IONP-PEG-Chitosan-DNA-CTX | MRI | [52] |
| MFNP-SiNP–CTX | MR/Optical imaging | [53] |
| IONP-PEG-Chitosan-Cy5.5-CTX | MR/Optical imaging | [54] |
| IONP-PEG-CTX | MR/Optical imaging | [55,56] |
| NaGdF4-Ho3+-CTX | MR/Optical imaging | [57] |
| Gd-DTPA/BODIPY-dendrigraft poly-L-lysines-PEG-CTX | MR/Optical imaging | [58] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin—A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins 2018, 10, 496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120496
Cohen G, Burks SR, Frank JA. Chlorotoxin—A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins. 2018; 10(12):496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120496
Chicago/Turabian StyleCohen, Gadi, Scott R. Burks, and Joseph A. Frank. 2018. "Chlorotoxin—A Multimodal Imaging Platform for Targeting Glioma Tumors" Toxins 10, no. 12: 496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120496




