Revisiting the Therapeutic Potential of Bothrops jararaca Venom: Screening for Novel Activities Using Connectivity Mapping
Abstract
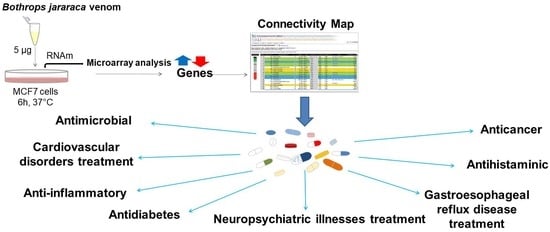
:1. Introduction
2. Results and Discussion
2.1. Gene Expression Analysis
2.2. Connectivity Map Analysis
2.2.1. Major Drug Classes Positively Correlated to Venom through C-Map Analysis
Antimicrobial Activity
Neuropsychiatric Illnesses
Cardiovascular Related Disorders
Anti-Inflammatory
Other Relevant Potential Applications
2.2.2. Major Drug Classes Negatively Correlated to Venom through C-Map Analysis
3. Conclusions
4. Materials and Methods
4.1. Venom
4.2. Tissue Culture
4.3. MCF7 Cells Treatment with B. jararaca Venom
4.4. Gene Expression Analysis
4.5. Bioinformatics Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Hrebickova, L.; Nawarskas, J.J.; Anderson, J.R. Ximelagatran: A new oral anticoagulant. Heart Dis. 2003, 5, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ciscotto, P.; Machado de Avila, R.A.; Coelho, E.A.; Oliveira, J.; Diniz, C.G.; Farias, L.M.; de Carvalho, M.A.; Maria, W.S.; Sanchez, E.F.; Borges, A.; et al. Antigenic, microbicidal and antiparasitic properties of an L-amino acid oxidase isolated from Bothrops jararaca snake venom. Toxicon 2009, 53, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, S.; Leiva, L.C.; Merino, L.; Acosta, O.; Joffé, E.B.d.K.; Gorodner, J.O. Antimicrobial activity of Bothrops alternatus venom from the Northeast of Argentine. Rev. Latinoam. Microbiol. 2008, 50, 79–82. [Google Scholar]
- Torres, A.F.C.; Dantas, R.T.; Menezes, R.R.P.P.B.; Toyama, M.H.; Oliveira, M.F.; Nogueira, N.A.P.; Oliveira, M.R.; Monteiro, H.S.A.; Martins, A.M.C. Antimicrobial activity of an l-amino acid oxidase isolated from Bothrops leucurus snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 614–622. [Google Scholar] [CrossRef]
- De Melo Alves Paiva, R.; de Freitas Figueiredo, R.; Antonucci, G.A.; Paiva, H.H.; de Lourdes Pires Bianchi, M.; Rodrigues, K.C.; Lucarini, R.; Caetano, R.C.; Linhari Rodrigues Pietro, R.C.; Gomes Martins, C.H.; et al. Cell cycle arrest evidence, parasiticidal and bactericidal properties induced by l-amino acid oxidase from Bothrops atrox snake venom. Biochimie 2011, 93, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Paramo, L.; Lomonte, B.; Pizarro-Cerda, J.; Bengoechea, J.A.; Gorvel, J.P.; Moreno, E. Bactericidal activity of Lys49 and Asp49 myotoxic phospholipases A2 from Bothrops asper snake venom—Synthetic Lys49 myotoxin II-(115-129)-peptide identifies its bactericidal region. Eur. J. Biochem. 1998, 253, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Stabeli, R.G.; Marcussi, S.; Carlos, G.B.; Pietro, R.C.; Selistre-de-Araujo, H.S.; Giglio, J.R.; Oliveira, E.B.; Soares, A.M. Platelet aggregation and antibacterial effects of an l-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorg. Med. Chem. 2004, 12, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, C.; Larios, S.; Quiros, S.; Pizarro-Cerda, J.; Gorvel, J.P.; Lomonte, B.; Moreno, E. Bactericidal and antiendotoxic properties of short cationic peptides derived from a snake venom Lys49 phospholipase A2. Antimicrob. Agents Chemother. 2005, 49, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; Andrade, H.F., Jr.; Spencer, P.J.; Lourenco, C.O.; Rogero, J.R.; Nascimento, N. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its L-amino acid oxidase. Biochem. Biophys. Res. Commun. 2001, 280, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Zieler, H.; Keister, D.B.; Dvorak, J.A.; Ribeiro, J.M. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J. Exp. Biol. 2001, 204, 4157–4167. [Google Scholar] [PubMed]
- Franca, S.C.; Kashima, S.; Roberto, P.G.; Marins, M.; Ticli, F.K.; Pereira, J.O.; Astolfi-Filho, S.; Stabeli, R.G.; Magro, A.J.; Fontes, M.R.; et al. Molecular approaches for structural characterization of Bothrops L-amino acid oxidases with antiprotozoal activity: CDNA cloning, comparative sequence analysis, and molecular modeling. Biochem. Biophys. Res. Commun. 2007, 355, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Deolindo, P.; Teixeira-Ferreira, A.S.; Melo, E.J.; Arnholdt, A.C.; Souza, W.; Alves, E.W.; DaMatta, R.A. Programmed cell death in Trypanosoma cruzi induced by Bothrops jararaca venom. Mem. Inst. Oswaldo Cruz 2005, 100, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Deolindo, P.; Teixeira-Ferreira, A.S.; DaMatta, R.A.; Alves, E.W. L-amino acid oxidase activity present in fractions of Bothrops jararaca venom is responsible for the induction of programmed cell death in Trypanosoma cruzi. Toxicon 2010, 56, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Vargas, L.J.; Segura, C.; Gutierrez, J.M.; Perez, J.C. In vitro antiplasmodial activity of phospholipases A2 and a phospholipase homologue isolated from the venom of the snake Bothrops asper. Toxins 2012, 4, 1500–1516. [Google Scholar] [CrossRef] [PubMed]
- Petricevich, V.L.; Mendonca, R.Z. Inhibitory potential of Crotalus durissus terrificus venom on measles virus growth. Toxicon 2003, 42, 143–153. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.C.; Lazdunski, M.; Doglio, A. Secreted phospholipases A(2), a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, J.H.; Lee, W.H.; Wang, Q.; Liu, H.; Zheng, Y.T.; Zhang, Y. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem. Biophys. Res. Commun. 2003, 309, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Bernardi, M.M.; Cury, Y. Analgesic effect evoked by low molecular weight substances extracted from Crotalus durissus terrificus venom. Toxicon 1993, 31, 1257–1265. [Google Scholar] [CrossRef]
- Pu, X.C.; Wong, P.T.; Gopalakrishnakone, P. A novel analgesic toxin (hannalgesin) from the venom of king cobra (Ophiophagus hannah). Toxicon 1995, 33, 1425–1431. [Google Scholar] [CrossRef]
- Mancin, A.C.; Soares, A.M.; Andriao-Escarso, S.H.; Faca, V.M.; Greene, L.J.; Zuccolotto, S.; Pela, I.R.; Giglio, J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon 1998, 36, 1927–1937. [Google Scholar] [CrossRef]
- Chen, Z.X.; Zhang, H.L.; Gu, Z.L.; Chen, B.W.; Han, R.; Reid, P.F.; Raymond, L.N.; Qin, Z.H. A long-form alpha-neurotoxin from cobra venom produces potent opioid-independent analgesia. Acta Pharmacol. Sin. 2006, 27, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Liang, Y.X.; Han, L.P.; Qiu, P.X.; Yuan, J.; Zhao, S.J. Purification and characterization of a novel antinociceptive toxin from Cobra venom (Naja naja atra). Toxicon 2008, 52, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Dhanak, A.C.; Rishipathak, D.D.; Gide, P.S. Multiple sclerosis & it´s treatment with alpha-cobratoxin: A review. Int. J. PharmTech Res. 2010, 2, 740–749. [Google Scholar]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Sundaram, M.S.; Kemparaju, K.; Girish, K.S. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon 2015, 98, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Nicolau, C.A.; Escalante, T.; Kim, J.; Herrera, C.; Gutierrez, J.M.; Fox, J.W. Viperid envenomation wound exudate contributes to increased vascular permeability via a DAMPs/TLR-4 mediated pathway. Toxins 2016, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.G.; Bao, Y.; Serrano, S.M.; Kamiguti, A.S.; Theakston, R.D.; Fox, J.W. Use of microarrays for investigating the subtoxic effects of snake venoms: Insights into venom-induced apoptosis in human umbilical vein endothelial cells. Toxicon 2003, 41, 429–440. [Google Scholar] [CrossRef]
- Gallagher, P.; Bao, Y.; Serrano, S.M.; Laing, G.D.; Theakston, R.D.; Gutierrez, J.M.; Escalante, T.; Zigrino, P.; Moura-da-Silva, A.M.; Nischt, R.; et al. Role of the snake venom toxin jararhagin in proinflammatory pathogenesis: In vitro and in vivo gene expression analysis of the effects of the toxin. Arch. Biochem. Biophys. 2005, 441, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W. Insights in to Venom and Toxin Activities and Pharmacological/Therapeutic Potential Using Gene Expression Profiling. In Toxins and Hemostasis from the Bench to Bedside; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 73–81. [Google Scholar]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J. The Connectivity Map: A new tool for biomedical research. Nat. Rev. Cancer 2007, 7, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Aramadhaka, L.R.; Prorock, A.; Dragulev, B.; Bao, Y.; Fox, J.W. Connectivity maps for biosimilar drug discovery in venoms: The case of Gila monster venom and the anti-diabetes drug Byetta(R). Toxicon 2013, 69, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, C.A.; Carvalho, P.C.; Junqueira-de-Azevedo, I.L.; Teixeira-Ferreira, A.; Junqueira, M.; Perales, J.; Neves-Ferreira, A.G.; Valente, R.H. An in-depth snake venom proteopeptidome characterization: Benchmarking Bothrops jararaca. J. Proteom. 2017, 151, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 2013, 62, 75–82. [Google Scholar] [CrossRef] [PubMed]
- McCleary, R.J.; Kini, R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon 2013, 62, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-Franca, J.; Cologna, C.T.; Pucca, M.B.; Bordon, K.C.; Amorim, F.G.; Anjolette, F.A.; Cordeiro, F.A.; Wiezel, G.A.; Cerni, F.A.; Pinheiro-Junior, E.L.; et al. Minor snake venom proteins: Structure, function and potential applications. Biochim. Biophys. Acta 2017, 1861, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, J.B.; Fox, J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Jia, Y.; Perez, J.C. Molecular cloning and characterization of cDNAs encoding metalloproteinases from snake venom glands. Toxicon 2010, 55, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon 2005, 45, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Clemetson, K.J. Reptile C-Type Lectins. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: New York, NY, USA, 2010; pp. 359–375. [Google Scholar]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Lodovicho, M.E.; Costa, T.R.; Bernardes, C.P.; Menaldo, D.L.; Zoccal, K.F.; Carone, S.E.; Rosa, J.C.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; et al. Investigating possible biological targets of Bj-CRP, the first cysteine-rich secretory protein (CRISP) isolated from Bothrops jararaca snake venom. Toxicol. Lett. 2017, 265, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Clemetson, K.J. Snake venom L-amino acid oxidases. Toxicon 2002, 40, 659–665. [Google Scholar] [CrossRef]
- Junqueira de Azevedo, I.L.; Farsky, S.H.; Oliveira, M.L.; Ho, P.L. Molecular cloning and expression of a functional snake venom vascular endothelium growth factor (VEGF) from the Bothrops insularis pit viper. A new member of the VEGF family of proteins. J. Biol. Chem. 2001, 276, 39836–39842. [Google Scholar] [CrossRef] [PubMed]
- Junqueira-de-Azevedo Ide, L.; da Silva, M.B.; Chudzinski-Tavassi, A.M.; Ho, P.L. Identification and cloning of snake venom vascular endothelial growth factor (svVEGF) from Bothrops erythromelas pitviper. Toxicon 2004, 44, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Camargo, A.C.; Ogawa, T.; Deshimaru, M.; Ohno, M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology 1999, 44, 129–135. [Google Scholar] [CrossRef]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Santoro, M.L.; Vaquero, T.S.; Leme, A.F.; Serrano, S.M. NPP-BJ, a nucleotide pyrophosphatase/phosphodiesterase from Bothrops jararaca snake venom, inhibits platelet aggregation. Toxicon 2009, 54, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Kemparaju, K.; Girish, K.S. Snake venom hyaluronidase: A therapeutic target. Cell Biochem. Funct. 2006, 24, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.H.; Dragulev, B.; Perales, J.; Fox, J.W.; Domont, G.B. BJ46a, a snake venom metalloproteinase inhibitor. Isolation, characterization, cloning and insights into its mechanism of action. Eur. J. Biochem. 2001, 268, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.W.; Muller-Eberhard, H.J. Cobra venom factor: Improved method for purification and biochemical characterization. J. Immunol. Methods 1984, 73, 203–220. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch. Biochem. Biophys. 2012, 522, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Kroemer, G. Mitochondria: Master regulators of danger signalling. Nat. Rev. Mol. Cell. Biol. 2012, 13, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kashyap, A.; Pandey, R.V.; Saini, K.S. Novel advances in cytochrome P450 research. Drug Discov. Today 2011, 16, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol. Ther. 2010, 125, 446–463. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.; Landucci, E.C.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.C.; Gammon, C.M.; Ousley, A.H.; McCarthy, K.D.; Morell, P. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. J. Neurochem. 1992, 58, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.A.; Dhanasekaran, S.M.; Tomlins, S. Immediate early inflammatory gene responses of human umbilical vein endothelial cells to hemorrhagic venom. Inflamm. Res. 2011, 60, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Seixas, E.; Gozzelino, R.; Chora, A.; Ferreira, A.; Silva, G.; Larsen, R.; Rebelo, S.; Penido, C.; Smith, N.R.; Coutinho, A.; et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl. Acad. Sci. USA 2009, 106, 15837–15842. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Petricevich, V.L.; Teixeira, C.F.; Tambourgi, D.V.; Gutierrez, J.M. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon 2000, 38, 1253–1266. [Google Scholar] [CrossRef]
- Chaves, F.; Teixeira, C.F.; Gutierrez, J.M. Role of TNF-alpha, IL-1beta and IL-6 in the local tissue damage induced by Bothrops asper snake venom: An experimental assessment in mice. Toxicon 2005, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Rucavado, A.; Chaves, F.; Diaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; von Minckwitz, G.; Weber, S.; Sinn, H.P.; Schini-Kerth, V.B.; Lobysheva, I.; Nepveu, F.; Wolf, G.; Strebhardt, K.; Kaufmann, M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer 2002, 95, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Bulut, A.S.; Erden, E.; Sak, S.D.; Doruk, H.; Kursun, N.; Dincol, D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: An immunohistochemical study of 151 cases. Virchows Arch. 2005, 447, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.C.; Agnihotri, S.; Mack, S.C.; Golbourn, B.J.; Diaz, R.J.; Olsen, S.; Bryant, M.; Bebenek, M.; Wang, X.; Bertrand, K.C.; et al. Identification of alsterpaullone as a novel small molecule inhibitor to target group 3 medulloblastoma. Oncotarget 2015, 6, 21718–21729. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, L.F.; Ribeiro, M.C.; Souza, G.R.; Sant’Ana, C.D.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Goulart, L.R.; Beleboni, R.O.; Nomizo, A.; Sampaio, S.V.; et al. Biochemical and functional characterization of an L-amino acid oxidase isolated from Bothrops pirajai snake venom. Bioorg. Med. Chem. 2006, 14, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, R.R.; Torres, A.F.; da Silva, T.S.; de Sousa, D.F.; Lima, D.B.; Norjosa, D.B.; Nogueira, N.A.; Oliveira, M.F.; de Oliveira, M.R.; Monteiro, H.S.; et al. Antibacterial and antiparasitic effects of Bothropoides lutzi venom. Nat. Prod. Commun. 2012, 7, 71–74. [Google Scholar] [PubMed]
- Goncalves, A.R.; Soares, M.J.; de Souza, W.; DaMatta, R.A.; Alves, E.W. Ultrastructural alterations and growth inhibition of Trypanosoma cruzi and Leishmania major induced by Bothrops jararaca venom. Parasitol. Res. 2002, 88, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Passero, L.F.; Laurenti, M.D.; Tomokane, T.Y.; Corbett, C.E.; Toyama, M.H. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol. Res. 2008, 102, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Grabner, A.N.; Alfonso, J.; Kayano, A.M.; Moreira-Dill, L.S.; Dos Santos, A.P.A.; Caldeira, C.A.S.; Sobrinho, J.C.; Gomez, A.; Grabner, F.P.; Cardoso, F.F.; et al. BmajPLA2-II, a basic Lys49-phospholipase A2 homologue from Bothrops marajoensis snake venom with parasiticidal potential. Int. J. Biol. Macromol. 2017, 102, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Carone, S.E.I.; Costa, T.R.; Burin, S.M.; Cintra, A.C.O.; Zoccal, K.F.; Bianchini, F.J.; Tucci, L.F.F.; Franco, J.J.; Torqueti, M.R.; Faccioli, L.H.; et al. A new l-amino acid oxidase from Bothrops jararacussu snake venom: Isolation, partial characterization, and assessment of pro-apoptotic and antiprotozoal activities. Int. J. Biol. Macromol. 2017, 103, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.P.; Lima, D.B.; Menezes, R.R.; Bandeira, I.C.; Tessarolo, L.D.; Sampaio, T.L.; Falcao, C.B.; Radis-Baptista, G.; Martins, A.M. Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon 2017, 130, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, C.; Deregnaucourt, C.; Clavey, V.; Schrevel, J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon 2004, 43, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.C.; Chacon, A.M.; Vargas, L.; Segura, C.; Gutierrez, J.M.; Alarcon, J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Morais, K.L.; Hayashi, M.A.; Bruni, F.M.; Lopes-Ferreira, M.; Camargo, A.C.; Ulrich, H.; Lameu, C. Bj-PRO-5a, a natural angiotensin-converting enzyme inhibitor, promotes vasodilatation mediated by both bradykinin B(2)and M1 muscarinic acetylcholine receptors. Biochem. Pharmacol. 2011, 81, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, C.A.; Kassel, O.; Mathieu, E.; Massard, G.; Gasser, B.; Frossard, N. Inhibition by glucocorticoids of the interleukin-1beta-enhanced expression of the mast cell growth factor SCF. Br. J. Pharmacol. 2002, 135, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Burin, S.M.; Menaldo, D.L.; de Castro, F.A.; Sampaio, S.V. Snake venom L-amino acid oxidases: An overview on their antitumor effects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ande, S.R.; Kommoju, P.R.; Draxl, S.; Murkovic, M.; Macheroux, P.; Ghisla, S.; Ferrando-May, E. Mechanisms of cell death induction by L-amino acid oxidase, a major component of ophidian venom. Apoptosis 2006, 11, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.Y.; Lee, M.L.; Tan, N.H. Molecular mechanism of cell death induced by king cobra (Ophiophagus hannah) venom l-amino acid oxidase. Toxicon 2015, 96, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Saviola, A.J.; Burns, P.D.; Mackessy, S.P. Apoptosis induction in human breast cancer (MCF-7) cells by a novel venom l-amino acid oxidase (Rusvinoxidase) is independent of its enzymatic activity and is accompanied by caspase-7 activation and reactive oxygen species production. Apoptosis 2015, 20, 1358–1372. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.M.; Santos, N.A.; Sartim, M.A.; Cintra, A.C.; Sampaio, S.V.; Santos, A.C. A tripeptide isolated from Bothrops atrox venom has neuroprotective and neurotrophic effects on a cellular model of Parkinson’s disease. Chem. Biol. Interact. 2015, 235, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Datta, P.; Das, T.; Biswas, A.K. Anti arthritic and anti inflammatory activity of a cytotoxic protein NN-32 from Indian spectacle cobra (Naja naja) venom in male albino rats. Toxicon 2014, 90, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Yao, L.; Zhang, B.; Zhang, S.; Guo, J. Anti-inflammatory effects of Neurotoxin-Nna, a peptide separated from the venom of Naja naja atra. BMC Complement. Altern. Med. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Folch, B.; Letourneau, M.; Vaudry, D.; Truong, N.H.; Doucet, N.; Chatenet, D.; Fournier, A. Cardiotoxin-I: An unexpectedly potent insulinotropic agent. Chembiochem 2012, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.W.; Bhat, V.K.; Flatt, P.R.; Gault, V.A.; McClean, S. Isolation and characterisation of insulin-releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis venom. Toxicon 2015, 101, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Toyama, O.D.; Boschero, C.A.; Martins, A.M.; Fonteles, C.M.; Monteiro, S.H.; Toyama, H.M. Structure-function relationship of new crotamine isoform from the Crotalus durissus cascavella. Protein J. 2005, 24, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lajus, S.; Vacher, P.; Huber, D.; Dubois, M.; Benassy, M.N.; Ushkaryov, Y.; Lang, J. Alpha-latrotoxin induces exocytosis by inhibition of voltage-dependent K+ channels and by stimulation of L-type Ca2+ channels via latrophilin in beta-cells. J. Biol. Chem. 2006, 281, 5522–5531. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nakaki, T.; Nakadate, T.; Kato, R. Insulinotropic effects of exogenous phospholipase A2 and C in isolated pancreatic islets. Eur. J. Pharmacol. 1982, 86, 121–124. [Google Scholar] [CrossRef]
- Nogueira, T.C.; Ferreira, F.; Toyama, M.H.; Stoppiglia, L.F.; Marangoni, S.; Boschero, A.C.; Carneiro, E.M. Characterization of the insulinotropic action of a phospholipase A2 isolated from Crotalus durissus collilineatus rattlesnake venom on rat pancreatic islets. Toxicon 2005, 45, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, G.; Franzoni, L.; de Chiara, C.; Mancin, A.C.; Giglio, J.R.; Spisni, A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur. J. Biochem. 2003, 270, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. Guidelines for Malaria Treatment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Baird, J.K.; Rieckmann, K.H. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003, 19, 115–120. [Google Scholar] [CrossRef]
- Price, R.N.; Douglas, N.M.; Anstey, N.M.; von Seidlein, L. Plasmodium vivax treatments: What are we looking for? Curr. Opin. Infect. Dis. 2011, 24, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Howes, R.E.; Battle, K.E.; Satyagraha, A.W.; Baird, J.K.; Hay, S.I. G6PD deficiency: Global distribution, genetic variants and primaquine therapy. Adv. Parasitol. 2013, 81, 133–201. [Google Scholar] [PubMed]
- Thomas, D.; Tazerouni, H.; Sundararaj, K.G.; Cooper, J.C. Therapeutic failure of primaquine and need for new medicines in radical cure of Plasmodium vivax. Acta Trop. 2016, 160, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Fitch, C.D.; Chevli, R.; Banyal, H.S.; Phillips, G.; Pfaller, M.A.; Krogstad, D.J. Lysis of Plasmodium falciparum by ferriprotoporphyrin IX and a chloroquine-ferriprotoporphyrin IX complex. Antimicrob. Agents Chemother. 1982, 21, 819–822. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, K.A.; Marques, H.M.; Egan, T.J. The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J. Inorg. Biochem. 2008, 102, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Vippagunta, S.R.; Matile, H.; Jaquet, C.; Vennerstrom, J.L.; Ridley, R.G. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 1998, 55, 727–736. [Google Scholar] [CrossRef]
- Werlinder, V.; Backlund, M.; Zhukov, A.; Ingelman-Sundberg, M. Transcriptional and post-translational regulation of CYP1A1 by primaquine. J. Pharmacol. Exp. Ther. 2001, 297, 206–214. [Google Scholar] [PubMed]
- Nagaraj, V.A.; Sundaram, B.; Varadarajan, N.M.; Subramani, P.A.; Kalappa, D.M.; Ghosh, S.K.; Padmanaban, G. Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 2013, 9, e1003522. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Sigala, P.A.; Miura, K.; Morrisey, J.M.; Mather, M.W.; Crowley, J.R.; Henderson, J.P.; Goldberg, D.E.; Long, C.A.; Vaidya, A.B. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J. Biol. Chem. 2014, 289, 34827–34837. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, A.; Ferreira, A.; Balla, J.; Jeney, V.; Balla, G.; Epiphanio, S.; Chora, A.; Rodrigues, C.D.; Gregoire, I.P.; Cunha-Rodrigues, M.; et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007, 13, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.D.; Martinelli, A.; Rodrigues, L.A.; do Carmo, E.L.; do Rosario, V.E.; Povoa, M.M.; Cravo, P. Plasmodium falciparum from Pará state (Brazil) shows satisfactory in vitro response to artemisinin derivatives and absence of the S769N mutation in the SERCA-type PfATPase6. Trop. Med. Int. Health 2008, 13, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, P.; Rattehalli, R.D.; Jayaram, M.B. Levomepromazine for schizophrenia. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Felder, C.C.; Tzavara, E.; Nomikos, G.G.; Calligaro, D.O.; McKinzie, D.L. Muscarinic mechanisms of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Thanacoody, R.H. Thioridazine: The good and the bad. Recent Pat. Anti-Infect. Drug Discov. 2011, 6, 92–98. [Google Scholar] [CrossRef]
- Jayakody, K.; Gibson, R.C.; Kumar, A.; Gunadasa, S. Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses. Cochrane Database Syst. Rev. 2012, 4, CD000525. [Google Scholar] [CrossRef] [PubMed]
- Tardy, M.; Huhn, M.; Engel, R.R.; Leucht, S. Fluphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014, 8, CD009230. [Google Scholar] [CrossRef] [PubMed]
- Selbie, L.A.; Hill, S.J. G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 1998, 19, 87–93. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Kaupmann, K. Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bettler, B.; Kaupmann, K.; Mosbacher, J.; Gassmann, M. Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 2004, 84, 835–867. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Sharma, S.; Kumar, P.; Deshmukh, R. Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol. Biochem. Behav. 2013, 110, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.C.; de Medeiros, W.A.; Batista Ide, F.; Pimenta, D.C.; Lebrun, I.; Abdalla, F.M.; Sandoval, M.R. Characterization of a new muscarinic toxin from the venom of the Brazilian coral snake Micrurus lemniscatus in rat hippocampus. Life Sci. 2011, 89, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.S.; Carsi-Gabrenas, J.; Krajewski, J.L.; McCafferty, J.M.; Purkerson, S.L.; Santiago, M.P.; Strauss, W.L.; Valentine, H.H.; Potter, L.T. Anti-muscarinic toxins from Dendroaspis angusticeps. Toxicon 1996, 34, 1257–1267. [Google Scholar] [CrossRef]
- Bradley, K.N. Muscarinic toxins from the green mamba. Pharmacol. Ther. 2000, 85, 87–109. [Google Scholar] [CrossRef]
- Karlsson, E.; Jolkkonen, M.; Mulugeta, E.; Onali, P.; Adem, A. Snake toxins with high selectivity for subtypes of muscarinic acetylcholine receptors. Biochimie 2000, 82, 793–806. [Google Scholar] [CrossRef]
- Servent, D.; Fruchart-Gaillard, C. Muscarinic toxins: Tools for the study of the pharmacological and functional properties of muscarinic receptors. J. Neurochem. 2009, 109, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Maiga, A.; Merlin, J.; Marcon, E.; Rouget, C.; Larregola, M.; Gilquin, B.; Fruchart-Gaillard, C.; Lajeunesse, E.; Marchetti, C.; Lorphelin, A.; et al. Orthosteric binding of rho-Da1a, a natural peptide of snake venom interacting selectively with the alpha1A-adrenoceptor. PLoS ONE 2013, 8, e68841. [Google Scholar] [CrossRef] [PubMed]
- Maiga, A.; Mourier, G.; Quinton, L.; Rouget, C.; Gales, C.; Denis, C.; Lluel, P.; Senard, J.M.; Palea, S.; Servent, D.; et al. G protein-coupled receptors, an unexploited animal toxin targets: Exploration of green mamba venom for novel drug candidates active against adrenoceptors. Toxicon 2012, 59, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Nareoja, K.; Nasman, J. Selective targeting of G-protein-coupled receptor subtypes with venom peptides. Acta Physiol. 2011, 204, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, G.; Upert, G.; Mourier, G.; Gilquin, B.; Gilles, N.; Servent, D. New alpha-adrenergic property for synthetic MTbeta and CM-3 three-finger fold toxins from black mamba. Toxicon 2013, 75, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, E.V.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N.; Vulfius, C.A. Viperidae Snake Venoms Block Nicotinic Acetylcholine Receptors and Voltage-Gated Ca2+ Channels in Identified Neurons of Fresh-Water Snail Lymnaea stagnalis. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2008, 2, 14–18. [Google Scholar]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Milelli, A.; Turrini, E.; Catanzaro, E.; Maffei, F.; Fimognari, C. Perspectives in Designing Multifunctional Molecules in Antipsychotic Drug Discovery. Drug Dev. Res. 2016, 77, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Horacek, J.; Bubenikova-Valesova, V.; Kopecek, M.; Palenicek, T.; Dockery, C.; Mohr, P.; Hoschl, C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006, 20, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed. Res. Int. 2014, 2014, 656370. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999, 21, 106S–115S. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Clinical studies on the mechanism of action of clozapine: The dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology 1989, 99, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol. Bull. 1988, 24, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Correll, C.U. The Role of Clozapine in Treatment-Resistant Schizophrenia. JAMA Psychiatry 2016, 73, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Fava, G.A.; Sonino, N. Mood and anxiety disorders as early manifestations of medical illness: A systematic review. Psychother. Psychosom. 2014, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sliz, D.; Hayley, S. Major depressive disorder and alterations in insular cortical activity: A review of current functional magnetic imaging research. Front. Hum. Neurosci. 2012, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Morilak, D.A.; Frazer, A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004, 7, 193–218. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [PubMed]
- Ye, Z.; Altena, E.; Nombela, C.; Housden, C.R.; Maxwell, H.; Rittman, T.; Huddleston, C.; Rae, C.L.; Regenthal, R.; Sahakian, B.J.; et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson’s disease. Brain 2014, 137, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Syvalahti, E.K.; Kunelius, R.; Lauren, L. Effects of antiparkinsonian drugs on muscarinic receptor binding in rat brain, heart and lung. Pharmacol. Toxicol. 1988, 62, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourao, R.H.; Lima-Dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef] [PubMed]
- Villard, E.; Soubrier, F. Molecular biology and genetics of the angiotensin-I-converting enzyme: Potential implications in cardiovascular diseases. Cardiovasc. Res. 1996, 32, 999–1007. [Google Scholar] [CrossRef]
- Cotton, J.; Hayashi, M.A.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo, A.C.; Dive, V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Fiszman, G.L.; Middonno, M.C.; de la Torre, E.; Farina, M.; Espanol, A.J.; Sales, M.E. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol. Ther. 2007, 6, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.L.; Sirois, P.; Tannock, I.F.; Chammas, R. The role of kinin receptors in cancer and therapeutic opportunities. Cancer Lett. 2014, 345, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Hoffman, B.B. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Chabner, B.A., Knollman, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2011; pp. 745–788. [Google Scholar]
- Rajagopal, V.; Kreitman, R.J. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the a2-macroglobulin receptor. J. Biol. Chem. 2000, 275, 7566–7573. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Pahari, S.; Hodgson, W.C.; Kini, R.M. Hypotensive agents from snake venoms. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2011, 59, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Horta, C.C.R.; Chatzaki, M.; Rezende, B.A.; Magalhães, B.F.; Duarte, C.G.; Felicori, L.F.; Oliveira-Mendes, B.B.R.; do Carmo, A.O.; hávez-Olórtegui, C.; Kalapothakis, E. Cardiovascular-Active Venom Toxins: An Overview. Curr. Med. Chem. 2016, 23, 603–622. [Google Scholar] [CrossRef]
- Accary, C.; Hraoui-Bloquet, S.; Sadek, R.; Alameddine, A.; Fajloun, Z.; Desfontis, J.C.; Mallem, Y. The relaxant effect of the Montivipera bornmuelleri snake venom on vascular contractility. J. Venom. Res. 2016, 7, 10–15. [Google Scholar] [PubMed]
- Santos, S.S.; Jesus, R.L.C.; Simoes, L.O.; Vasconcelos, W.P.; Medeiros, I.A.; Veras, R.C.; Casais, E.S.L.L.; Silva, D.F. NO production and potassium channels activation induced by Crotalus durissus cascavella underlie mesenteric artery relaxation. Toxicon 2017, 133, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; dos Santos, M.C.; Furtado Mde, F.; de Freitas, M.C.; da Silva, W.D.; Kipnis, T.L. Pro-inflammatory activities in elapid snake venoms. Br. J. Pharmacol. 1994, 112, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.P.; Fernandes, C.M.; Zuliani, J.P.; Zamuner, S.F. Inflammatory effects of snake venom metalloproteinases. Mem. Inst. Butantan 2005, 100, 181–184. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.C.; Rodrigues, R.S.; Lucena, M.N.; Cologna, C.T.; Oliveira, A.C.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Arantes, E.C.; Teixeira, D.N.; Ueira-Vieira, C.; et al. Isolation and functional characterization of proinflammatory acidic phospholipase A2 from Bothrops leucurus snake venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 154, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Fernandez, P.; Guillen, M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Choi, A.M. Heme oxygenase: Colors of defense against cellular stress. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000, 279, L1029–L1037. [Google Scholar] [CrossRef] [PubMed]
- Gullotta, F.; di Masi, A.; Coletta, M.; Ascenzi, P. CO metabolism, sensing, and signaling. Biofactors 2012, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Green, C.J.; Foresti, R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid. Redox Signal. 2002, 4, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, W.G.; Li, D.P.; Kung, H.; Lin, M.C. Heme oxygenase-1 system and gastrointestinal inflammation: A short review. World J. Gastroenterol. 2011, 17, 4283–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegiel, B.; Otterbein, L.E. Go green: The anti-inflammatory effects of biliverdin reductase. Front. Pharmacol. 2012, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Kumar, S. Snake venom: A potent anticancer agent. Asian Pac. J. Cancer Prev. 2012, 13, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.; Castro, R.; Lundin, C.; Hofstetter, A.; Alaniz, A.; Suntravat, M.; Sanchez, E.E. Inhibition of pancreatic tumoral cells by snake venom disintegrins. Toxicon 2015, 93, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; Sivashankari, P.R. Snake venom derived molecules in tumor angiogenesis and its application in cancer therapy; an overview. Curr. Top. Med. Chem. 2015, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, C.S.; Chittiboyina, A.G.; Kurtz, T.W.; Pershadsingh, H.A.; Avery, M.A. Type 2 diabetes and oral antihyperglycemic drugs. Curr. Med. Chem. 2008, 15, 61–74. [Google Scholar] [PubMed]
- Staels, B.; Fruchart, J. Therapeutic Roles of Peroxisome Proliferator—Activated Receptor Agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- American Association Diabetes. Standards of medical care in diabetes—2009. Diabetes Care 2009, 32 (Suppl. 1), S13–S61. [Google Scholar]
- Chandra, R.; Liddle, R.A. Neural and hormonal regulation of pancreatic secretion. Curr. Opin. Gastroenterol. 2009, 25, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, S.R.; Poitout, V. G protein-coupled receptors and insulin secretion: 119 and counting. Endocrinology 2007, 148, 2598–2600. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.E., III. The Role of Ion Channels in Insulin Secretion. J. Cell. Biochem. 1992, 48, 234–241. [Google Scholar] [CrossRef]
- Fridlyand, L.E.; Jacobson, D.A.; Philipson, L.H. Ion channels and regulation of insulin secretion in human β-cells: A computational systems analysis. Islets 2013, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, C.M.; Sanchez-Soto, C.; Hiriart, M. Toxins that modulate ionic channels as tools for exploring insulin secretion. Cell. Mol. Neurobiol. 2010, 30, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Barbosa, R.L.; Corso, G.; Boschero, A.C. Biochemical characterization of two crotamine isoforms isolated by a single step RP-HPLC from Crotalus durissus terrificus (South American rattlesnake) venom and their action on insulin secretion by pancreatic islets. Biochim. Biophys. Acta 2000, 1474, 56–60. [Google Scholar] [CrossRef]
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 1992, 267, 7402–7405. [Google Scholar] [PubMed]
- Furman, B.L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 2012, 59, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.H.; Chen, H.Q.; Veluthakal, R.; Silver, R.B.; Li, J.; Li, G.; Kowluru, A. Mastoparan-induced insulin secretion from insulin-secreting betaTC3 and INS-1 cells: Evidence for its regulation by Rho subfamily of G proteins. Endocrinology 2003, 144, 4508–4518. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Ribeiro, R.A.; Arcuri, H.A.; Palma, M.S.; Carneiro, E.M. Agelaia MP-I: A peptide isolated from the venom of the social wasp, Agelaia pallipes pallipes, enhances insulin secretion in mice pancreatic islets. Toxicon 2012, 60, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, H.; Deveney, C.W.; Bartholomew, C.; Raghupathy, E. Action of the venom of the scorpion Tityus trinitatis on pancreatic insulin secretion. Biochem. Pharmacol. 1983, 32, 1101–1104. [Google Scholar] [CrossRef]
- Goncalves, A.A.; Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Arantes, E.C.; Giglio, J.R.; Boschero, A.C. Participation of Na+ channels in the potentiation by Tityus serrulatus alpha-toxin TsTx-V of glucose-induced electrical activity and insulin secretion in rodent islet beta-cells. Toxicon 2003, 41, 1039–1045. [Google Scholar] [CrossRef]
- Holz, G.G.; Habener, J.F. Black widow spider alpha-latrotoxin: A presynaptic neurotoxin that shares structural homology with the glucagon-like peptide-1 family of insulin secretagogic hormones. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 121, 177–184. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Imani, S.; Haghighi, S.; Mousavi, S.E.; Karimi, A. Effect of Iranian Honey bee (Apis mellifera) Venom on Blood Glucose and Insulin in Diabetic Rats. J. Arthropod-Borne Dis. 2013, 6, 136–143. [Google Scholar]
- Vela, M.F. Medical treatments of GERD: The old and new. Gastroenterol. Clin. N. Am. 2014, 43, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, K.; Kinoshita, Y.; Habu, Y.; Oshima, T.; Manabe, N.; Fujiwara, Y.; Nagahara, A.; Kawamura, O.; Iwakiri, R.; Ozawa, S.; et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J. Gastroenterol. 2016, 51, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Gastroesophageal reflux disease (GERD): A review of conventional and alternative treatments. Altern. Med. Rev. 2011, 16, 116–133. [Google Scholar] [PubMed]
- Gremse, D.A. Lansoprazole: Pharmacokinetics, pharmacodynamics and clinical uses. Expert Opin. Pharmacother. 2001, 2, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Krusekopf, S.; Kleeberg, U.; Hildebrandt, A.G.; Ruckpaul, K. Effects of benzimidazole derivatives on cytochrome P450 1A1 expression in a human hepatoma cell line. Xenobiotica 1997, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Curi-Pedrosa, R.; Daujat, M.; Pichard, L.; Ourlin, J.C.; Clair, P.; Gervot, L.; Lesca, P.; Domergue, J.; Joyeux, H.; Fourtanier, G.; et al. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J. Pharmacol. Exp. Ther. 1994, 269, 384–392. [Google Scholar] [PubMed]
- Van der Pol, R.; Langendam, M.; Benninga, M.; van Wijk, M.; Tabbers, M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014, 168, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Galvao Nascimento, N.; Sampaio, M.C.; Amaral Olivo, R.; Teixeira, C. Contribution of mast cells to the oedema induced by Bothrops moojeni snake venom and a pharmacological assessment of the inflammatory mediators involved. Toxicon 2010, 55, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E. The antiallergic effects of antihistamines (H1-receptor antagonists). J. Allergy Clin. Immunol. 1992, 90, 705–715. [Google Scholar] [CrossRef]
- Stojković, N.; Cekić, S.; Ristov, M.; Ristić, M.; Đukić, D.; Binić, M.; Virijević, D. Histamine and Antihistamines. Sci. J. Fac. Med. 2015, 32, 7–22. [Google Scholar]
- Xie, H.; He, S.H. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J. Gastroenterol. 2005, 11, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Damerau, B.; Lege, L.; Oldigs, H.D.; Vogt, W. Histamine release, formation of prostaglandin-like activity (SRS-C) and mast cell degranulation by the direct lytic factor (DLF) and phospholipase A of cobra venom. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1975, 287, 141–156. [Google Scholar] [CrossRef]
- Chen, I.J.; Chiu, H.F.; Huang, H.T.; Teng, C.M. Edema formation and degranulation of mast cells by Trimeresurus mucrosquamatus snake venom. Toxicon 1984, 22, 17–28. [Google Scholar] [CrossRef]
- Wei, J.F.; Mo, Y.Z.; Qiao, L.Y.; Wei, X.L.; Chen, H.Q.; Xie, H.; Fu, Y.L.; Wang, W.Y.; Xiong, Y.L.; He, S.H. Potent histamine-releasing activity of atrahagin, a novel snake venom metalloproteinase. Int. J. Biochem. Cell Biol. 2006, 38, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, A.G.; da Costa, A.S.; Pires, A.L.; Neves-Ferreira, A.G.; Perales, J.; Cordeiro, R.S.; Martins, M.A.; e Silva, P.M. Contribution of mast cells and snake venom metalloproteinases to the hyperalgesia induced by Bothrops jararaca venom in rats. Toxicon 2006, 47, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.A.; Rivera, E.S. Histamine receptors and cancer pharmacology. Br. J. Pharmacol. 2010, 161, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Corboz, M.R.; Mutter, J.C.; Rivelli, M.A.; Mingo, G.G.; McLeod, R.L.; Varty, L.; Jia, Y.; Cartwright, M.; Hey, J.A. α2-adrenoceptor agonists as nasal decongestants. Pulm. Pharmacol. Ther. 2007, 20, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Au, L.C.; Huang, T.F. Molecular cloning and sequence analysis of aggretin, a collagen-like platelet aggregation inducer. Biochem. Biophys. Res. Commun. 1999, 263, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Lin, K.T.; Chang, C.H.; Peng, H.C.; Huang, T.F. The integrin alpha2beta1 agonist, aggretin, promotes proliferation and migration of VSMC through NF-kB translocation and PDGF production. Br. J. Pharmacol. 2009, 156, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]


| Protein Class | Associated Activities | Molecular Mass (kDa) | Relative Abundance (%) a |
|---|---|---|---|
| Metalloendopeptidase | Degrades extracelular matrix and coagulation cascade components leading to hemorrhage, edema, inflammation, and necrosis [38,39,40] | 20–100 | 33.6 |
| Serine endopeptidase | Acts on platelet aggregation, blood coagulation, fibrinolysis, complement system, blood pressure, and the nervous system [41,42,43] | 20–70 | 22.8 |
| C-type lectin/C-type lectin-like | Anticoagulant, procoagulant, agonist/antagonist of platelet activation [44] | 26–124 | 18.2 |
| Cysteine-rich secretory protein | Induces inflammatory response and affects the complement system (anaphylatoxins generation) [45,46] | 25 | 8.2 |
| Phospholipase A2 | Miotoxicity, neurotoxicity, anticoagulant effects [41,47] | 12–15 | 6.3 |
| l-amino acid oxidase | Agonist and antagonist of platelet aggregation; induces apoptosis [48] | 110–150 | 5.0 |
| Snake venom vascular endothelial growth factor | Increases vascular permeability [49,50] | 30 | 1.4 |
| Bradykinin-potentiating- and C-type-natriuretic peptides | Vasodilatation by inhibition of angiotensin-converting enzyme [1,51] | <2.5 | 1.3 |
| Phosphodiesterase | Pyrimidine and purine release, possibly contributing to the increase of vascular permeability [52,53] | 100–130 | <1.0 |
| Hyaluronidase | Degrades the hyaluronic acid present in the extracellular matrix, facilitating toxin diffusion [54] | 30–80 | <1.0 |
| Ecto-5′-nucleotidase | Pyrimidine and purines release, possibly contributing to the increase of vascular permeability [52] | 74 | <1.0 |
| Metalloendopeptidase inhibitor | Inhibits enzymatic and hemorrhagic activity of snake venom metalloendopeptidases; abundantly found in the snake’s plasma (protective mechanism) [55] | 46 | <1.0 |
| Disintegrin | Inhibits platelet aggregation [56] | 4–15 | <1.0 |
| Cobra venom factor b | Activates the complement cascade [57] | 149 | <1.0 |
| Three-finger toxin b | Neurotoxicity and cardiotoxicity effects by targeting nicotinic and muscarinic acetylcholinesterase receptors, beta-adrenergic receptors, and L-type calcium channels [58,59] | 6–8 | <1 |
| Activity | Venom Source | Reference |
|---|---|---|
| Antibacterial | Bothrops jararaca; B. asper; B. alternatus; B. atrox; B. pirajai: Bothropoides lutzi | [4,8,9,10,77,78] |
| Anti-parasatic (trypano-, leishmani-, and plasmodicidal) | B.jararaca; B. moojeni; Crotalus adamanteus; B. jararacussu; B. asper; B. pirajai; C. durissus collilineatus; B. marajoensis; B. lutzi; C. d. cumanensis | [4,11,12,13,14,16,77,78,79,80,81,82,83,84,85] |
| Antihypertensive | B. jararaca | [1,2,86] |
| Antitumor | B. jararaca; Ophiophagus hannah; Agkistrodon acutus; Bungarus fasciatus; B. atrox; B. leucurus; C. atrox; Lachesis muta; A. contortrix laticinctus; A. halys; A. halys pallas; B. moojeni; B. pirajai; Calloselasma rhodostoma | [87,88,89,90,91] |
| Antiparkinsonian | B. atrox | [92] |
| Anti-inflammatory and/or analgesic | Naja naja; N. n. atra; C. d. terrificus; O. hannah | [20,21,22,23,24,93,94] |
| Antidiabetic | C. adamanteus; C. vegrandis; Bitis nasico; C. d. cascavella; C. d. terrificus; N. kaouthia; C. d. collilineatus | [95,96,97,98,99,100,101] |
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Primaquine | 0.9 × 104 | 0.915 | 0.429 | −0.517 | Antiparasite (antimalarian activity) |
| Tanespimycin | 0.1 × 104 | 0.814 | 0.161 | −0.681 | Antineoplastic Antibiotic |
| Cefalonium | 0.9 × 104 | 0.775 | 0.250 | −0.551 | Antibiotic |
| Chlorhexidine | 0.8 × 104 | 0.743 | 0.102 | −0.667 | Antibiotic |
| Novobiocin | 1.0 × 105 | 0.737 | 0.056 | −0.706 | Antibiotic |
| Clioquinol | 1.3 × 104 | 0.737 | 0.366 | −0.396 | Antifungal and antiprotozoal |
| Erythromycin | 0.5 × 104 | 0.721 | 0.119 | −0.627 | Antibiotic |
| Tetracycline | 0.8 × 104 | 0.710 | 0.211 | −0.523 | Antibiotic |
| Piperacillin | 0.7 × 104 | 0.677 | 0.130 | −0.571 | Antibiotic |
| Ciclacillin | 1.2 × 104 | 0.675 | 0.069 | -0.629 | Antibiotic |
| Halofantrine | 0.7 × 104 | 0.675 | 0.146 | −0.552 | Antimalarial |
| Colistin | 0.3 × 104 | 0.665 | 0.167 | −0.521 | Antibiotic |
| Cefoxitin | 0.9 × 104 | 0.662 | 0.260 | −0.424 | Antibiotic |
| Minocycline | 1.1 × 104 | 0.653 | 0.143 | −0.532 | Antibiotic |
| Azlocillin | 0.8 × 104 | 0.649 | 0.099 | −0.572 | Antibiotic |
| Vancomycin | 0.3 × 104 | 0.646 | 0.096 | −0.572 | Antibiotic |
| Sulfamonomethoxine | 1.4 × 104 | 0.628 | 0.095 | −0.555 | Antibiotic |
| Dicloxacillin | 0.8 × 104 | 0.627 | 0.097 | −0.551 | Antibiotic |
| Hycanthone | 1.1 × 104 | 0.619 | 0.137 | −0.503 | Antischistosomal |
| Ribostamycin | 0.7 × 104 | 0.605 | 0.126 | −0.500 | Antibiotic |
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Carbamazepine | 1.0 × 102 | 0.803 | 0.219 | −0.611 | Anticonvulsant (epilepsy and nerve pain treatment) |
| Thioridazine | 1.0 × 104 | 0.802 | 0.241 | −0.589 | Antipsychotic (schizophrenia treatment) |
| Prochlorperazine | 1.0 × 104 | 0.787 | 0.218 | −0.596 | Antipsychotic (schizophrenia, nonpsychotic anxiety treatment) |
| Perphenazine | 1.0 × 104 | 0.785 | 0.260 | −0.552 | Antipsychotic (schizophrenia treatment) |
| Metixene | 1.2 × 104 | 0.778 | 0.302 | −0.503 | Antiparkinsonian |
| Pirlindole | 1.2 × 104 | 0.740 | 0.140 | −0.625 | Antidepressant |
| Mianserin | 1.3 × 104 | 0.726 | 0.226 | −0.525 | Antidepressant |
| Lisuride | 1.2 × 104 | 0.722 | 0.242 | −0.505 | Antiparkinsonian |
| Mesoridazine | 0.7 × 104 | 0.721 | 0.109 | −0.637 | Antipsychotic (schizophrenia treatment) |
| Clozapine | 1.0 × 104 | 0.712 | 0.109 | −0.627 | Antipsychotic (treatment-resistant schizophrenia) |
| Trimethadione | 2.8 × 104 | 0.681 | 0.117 | −0.587 | Anticonvulsant (seizures treatment) |
| Zuclopenthixol | 0.9 × 104 | 0.673 | 0.192 | −0.505 | Antipsychotic (schizophrenia treatment) |
| Haloperidol | 1.0 × 104 | 0.669 | 0.057 | −0.635 | Antipsychotic (schizophrenia and Huntington’s disease treatment) |
| Thioproperazine | 0.6 × 104 | 0.658 | 0.160 | −0.520 | Antipsychotic (schizophrenia treatment) |
| Diclofenamide | 1.3 × 104 | 0.631 | 0.141 | −0.511 | Anticonvulsant (antiglaucoma, antiepileptic) |
| Levomepromazine | 0.9 × 104 | 0.627 | 0.214 | −0.434 | Antipsychotic (schizophrenia, anxiety treatment) |
| Fluphenazine | 1.0 × 104 | 0.623 | 0.284 | −0.361 | Antipsychotic (psychotic disorders treatment) |
| Valproic Acid | 5.0 × 104 | 0.622 | 0.174 | −0.469 | Anticonvulsant (antiepileptic) |
| Paroxetine | 0.1 × 104 | 0.604 | 0.103 | −0.522 | Antidepressant |
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Clopamide | 1.2 × 104 | 0.771 | 0.126 | −0.671 | Antihypertensive |
| Dobutamine | 1.2 × 104 | 0.714 | 0.126 | −0.612 | Treatment of heart failure and cardiogenic shock |
| Amrinone | 2.1 × 104 | 0.709 | 0.137 | −0.596 | Vasodilator |
| Quinidine | 1.1 × 104 | 0.702 | 0.112 | −0.614 | Arrhythmias |
| Sotalol | 1.3 × 104 | 0.679 | 0.121 | −0.582 | Arrhythmias |
| Metolazone | 1.1 × 104 | 0.673 | 0.115 | −0.581 | Antihypertensive |
| Papaverine | 1.1 × 104 | 0.652 | 0.133 | −0.542 | Vasodilator |
| Phenoxybenzamine | 1.2 × 104 | 0.637 | 0.247 | −0.413 | Antihypertensive |
| Midodrine | 1.4 × 104 | 0.625 | 0.155 | −0.492 | Antihypotensive |
| Isoprenaline | 1.6 × 104 | 0.617 | 0.148 | −0.490 | Bradycardia |
| Minoxidil | 1.9 × 104 | 0.608 | 0.092 | −0.537 | Vasodilator |
| Moracizine | 0.9 × 104 | 0.607 | 0.198 | −0.430 | Arrhythmias |
| Hydroflumethiazide | 1.2 × 104 | 0.604 | 0.131 | −0.494 | Antihypertensive |
| Tocainide | 1.7 × 104 | 0.602 | 0.150 | −0.472 | Arrhythmias |
| Practolol | 1.5 × 104 | 0.602 | 0.152 | −0.471 | Arrhythmias |
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug type |
|---|---|---|---|---|---|
| Sulindac | 1.1 × 104 | 0.854 | 0.330 | −0.553 | Anti-inflammatory |
| Thalidomide | 1.0 × 105 | 0.752 | 0.145 | −0.632 | Anti-inflammatory |
| Oxyphenbutazone | 1.2 × 104 | 0.732 | 0.258 | −0.499 | Anti-inflammatory |
| Tenoxicam | 1.2 × 104 | 0.710 | 0.107 | −0.627 | Anti-inflammatory |
| Epirizole | 1.7 × 104 | 0.700 | 0.152 | −0.572 | Anti-inflammatory |
| Indoprofen | 1.4 × 104 | 0.672 | 0.071 | −0.624 | Anti-inflammatory and analgesic |
| Budesonide | 0.9 × 104 | 0.665 | 0.126 | −0.562 | Anti-inflammatory (Crohn’s Disease Treatment) |
| Methylprednisolone | 1.1 × 104 | 0.663 | 0.142 | −0.544 | Anti-inflammatory |
| Mefenamic Acid | 1.7 × 104 | 0.645 | 0.104 | −0.563 | Anti-inflammatory |
| Felbinac | 1.9 × 104 | 0.642 | 0.151 | −0.513 | Anti-inflammatory (analgesic and antipyretic) |
| Acemetacin | 1.0 × 104 | 0.627 | 0.116 | −0.532 | Anti-inflammatory |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau, C.A.; Prorock, A.; Bao, Y.; Neves-Ferreira, A.G.d.C.; Valente, R.H.; Fox, J.W. Revisiting the Therapeutic Potential of Bothrops jararaca Venom: Screening for Novel Activities Using Connectivity Mapping. Toxins 2018, 10, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020069
Nicolau CA, Prorock A, Bao Y, Neves-Ferreira AGdC, Valente RH, Fox JW. Revisiting the Therapeutic Potential of Bothrops jararaca Venom: Screening for Novel Activities Using Connectivity Mapping. Toxins. 2018; 10(2):69. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020069
Chicago/Turabian StyleNicolau, Carolina Alves, Alyson Prorock, Yongde Bao, Ana Gisele da Costa Neves-Ferreira, Richard Hemmi Valente, and Jay William Fox. 2018. "Revisiting the Therapeutic Potential of Bothrops jararaca Venom: Screening for Novel Activities Using Connectivity Mapping" Toxins 10, no. 2: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020069