Fusarium graminearum in Stored Wheat: Use of CO2 Production to Quantify Dry Matter Losses and Relate This to Relative Risks of Zearalenone Contamination under Interacting Environmental Conditions
Abstract
:1. Introduction
2. Results
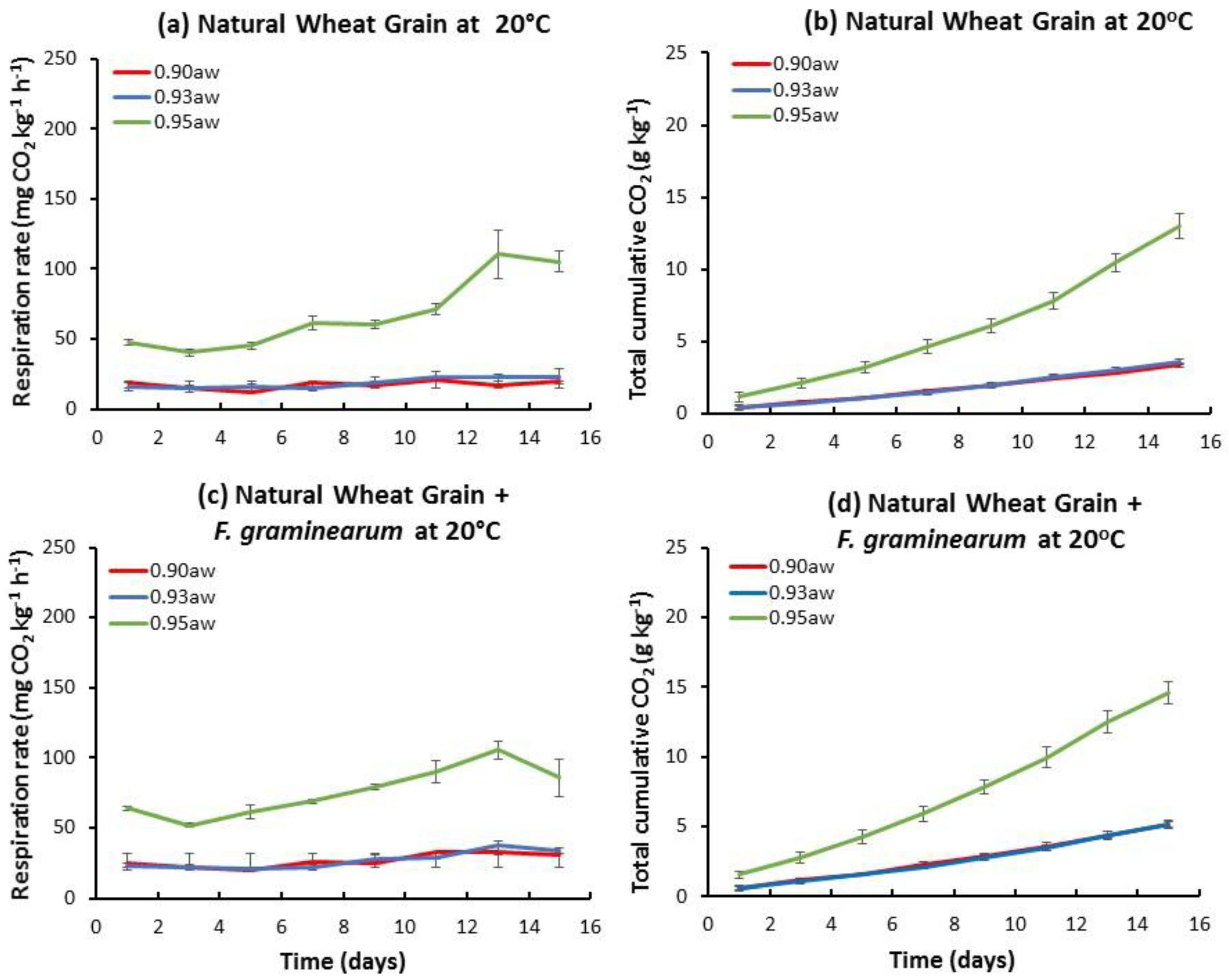
2.1. Influence of aw and Temperature on the Temporal Respiration Rate and Total CO2 Production in Stored Natural and Irradiated Wheat Grain with and without F. graminearum Inoculation
2.2. Relationship between Storage Environmental Conditions and Dry Matter Losses (DMLs) in Stored Natural/Irradiated Wheat and That Inoculated with F. graminearum
2.3. Zearalenone and Its Associated Compounds in Stored Wheat Inoculated with F. graminearum
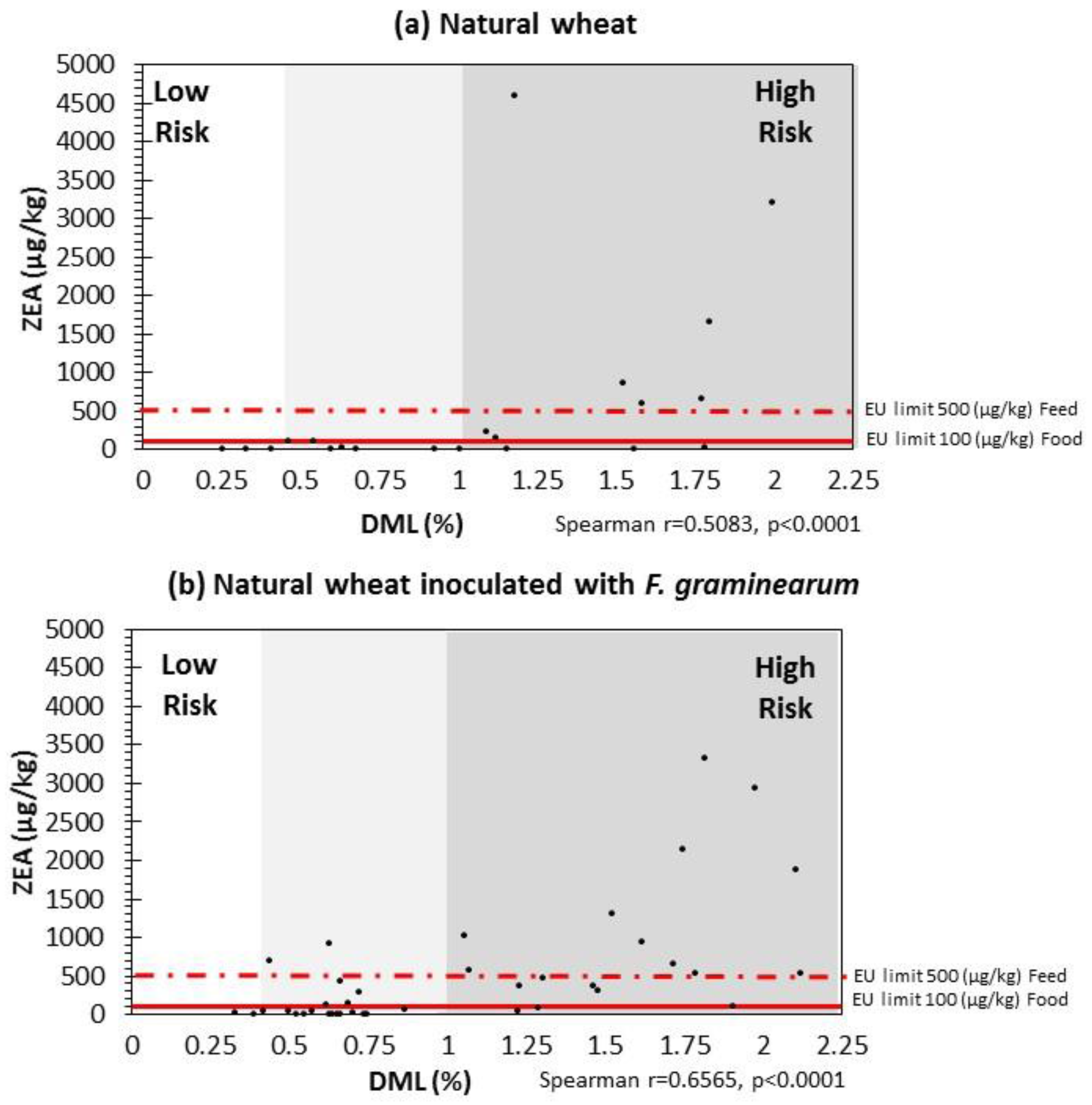
2.4. Correlation between DMLs and ZEN Contamination Relevant to EU Legislative Limits
3. Discussion
4. Materials and Methods
4.1. Fungal Culture
4.2. Wheat Grain Treatment, Moisture Content and Water Activity Adjustments
4.3. Grain Inoculation and Incubation
4.4. Respiration Measurement by Gas Chromatography (GC)
4.5. Mycotoxin Analyses
4.5.1. Sample Preparation
4.5.2. LC-MS/MS Parameters
4.6. Statistical Analysis and Modelling the Results
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiménez, M.; Máñez, M.; Hernández, E. Influence of water activity and temperature on the production of zearalenone in corn by three Fusarium species. Int. J. Food Microbiol. 1996, 29, 417–421. [Google Scholar] [CrossRef]
- Llorens, A.; Mateo, R.; Hinojo, M.J.; Logrieco, A.; Jimenez, M. Influence of the interactions among ecological variables in the characterization of zearalenone producing isolates of Fusarium spp. Syst. Appl. Microbiol. 2004, 27, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kanora, A.; Maes, D. The role of mycotoxins in pig reproduction: A review. Vet. Med. 2009, 54, 565–576. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC). No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commun. 2006, L364, 5–24. [Google Scholar] [CrossRef]
- European Commission Commission Recomendation (EC). No 576/2006 of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Commun. 2006, L229, 7–9. [Google Scholar]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A 2010, 27. [Google Scholar] [CrossRef] [PubMed]
- Mylona, K.; Sulyok, M.; Magan, N. Relationship between environmental factors, dry matter loss and mycotoxin levels in stored wheat and maize infected with Fusarium species. Food Addit. Contam. Part A 2012, 29. [Google Scholar] [CrossRef] [PubMed]
- Martín Castaño, S.; Medina, A.; Magan, N. Comparison of dry matter losses and aflatoxin B1 contamination of paddy and brown rice stored naturally or after inoculation with Aspergillus flavus at different environmental conditions. J. Stored Prod. Res. 2017, 73, 47–53. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, V.; Magan, N. In Mycotoxins in food: Detection and control. In Environmental Profiles for Growth and Mycotoxin Production; Magan, N., Olsen, M., Eds.; Woodhead Publishing Ltd.: Abingdon, Cambridge, UK, 2004; pp. 174–189. [Google Scholar]
- Mylona, K.; Magan, N. Fusarium langsethiae: Storage environment influences dry matter losses and T2 and HT-2 toxin contamination of oats. J. Stored Prod. Res. 2011, 47. [Google Scholar] [CrossRef]
- Kaleta, A.; Górnicki, K. Criteria of determination of safe grain storage time. In A Review, Advances in Agrophysical Research; Grundas, S., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- White, N.D.G.; Sinha, R.N.; Muir, W.E. Intergranuar carbon dioxide as an indicator of biological activity associated with the spoilage of stored wheat. CBE J. 1982, 24, 35–42. [Google Scholar]
- Lacey, J.; Hamer, A.; Magan, N. Respiration and dry matter losses in wheat grain under different environmental factors. In In Stored Product Protection; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Canberra, Australia, 1994; pp. 1007–1013. [Google Scholar]
- Seitz, L.M.; Sauer, D.B.; Mohr, H.E.; Aldis, D.F. Fungal growth and dry matter loss during bin storage of high-moisture corn. Cereal Chem. J. 1982, 59, 9–14. [Google Scholar]
- Garcia, D.; Garcia-Cela, E.; Ramos, A.J.; Sanchis, V.; Marín, S. Mould growth and mycotoxin production as affected by Equisetum arvense and Stevia rebaudiana extracts. Food Control 2011, 22, 1378–1384. [Google Scholar] [CrossRef]
- Velluti, A.; Marı́n, S.; Bettucci, L.; Ramos, A.; Sanchis, V. The effect of fungal competition on colonization of maize grain by Fusarium moniliforme, F. proliferatum and F. graminearum and on fumonisin B1 and zearalenone formation. Int. J. Food Microbiol. 2000, 59, 59–66. [Google Scholar] [CrossRef]
- Velluti, A.; Marín, S.; Gonzalez, R.; Ramos, A.J.; Sanchis, V. Fumonisin B1, zearalenone and deoxynivalenol production by Fusarium moniliforme, F proliferatum and F graminearum in mixed cultures on irradiated maize kernels. J. Sci. Food Agric. 2001, 81, 88–94. [Google Scholar] [CrossRef]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Saïd, S.; Sanchis, V. Influence of temperature, water activity and incubation time on fungal growth and production of ochratoxin A and zearalenone by toxigenic Aspergillus tubingensis and Fusarium incarnatum isolates in sorghum seeds. Int. J. Food Microbiol. 2017, 242, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Velluti, A.; Sanchis, V.; Ramos, A.J.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, M. Aflatoxin B1, zearalenone and deoxynivalenol production by Aspergillus parasiticus and Fusarium graminearum in interactive cultures on irradiated corn kernels. Mycopathologia 1998, 142, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Armando, M.R.; Dogi, C.A.; Poloni, V.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. In vitro study on the effect of Saccharomyces cerevisiae strains on growth and mycotoxin production by Aspergillus carbonarius and Fusarium graminearum. Int. J. Food Microbiol. 2013, 161, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Castaño, S.M.; Medina, A.; Magan, N. Impact of storage environment on respiration, dry matter losses and fumonisin B1 contamination of stored paddy and brown rice. World Mycotoxin J. 2017, 10, 319–326. [Google Scholar] [CrossRef]
- Bresler, G.; Vaamonde, G.; Degrossi, C.; Fernandez Pinto, V. Amaranth grain as substrate for aflatoxin and zearalenone production at different water activity levels. Int. J. Food Microbiol. 1998, 42, 57–61. [Google Scholar] [CrossRef]
- Greenhalgh, R.; Neish, G.A.; Miller, J.D. Deoxynivalenol, acetyl deoxynivalenol, and zearalenone formation by Canadian isolates of Fusarium graminearum on solid substrates. Appl. Environ. Microbiol. 1983, 46, 625–629. [Google Scholar] [PubMed]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Magan, N. Interacting environmental stress factors affects targeted metabolomic profiles in stored natural wheat and that inoculated with F. graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.D.; Lopez, T.A. Influence of temperature on zearalenone production by regional strains of Fusarium graminearum and Fusarium oxysporum in culture. Int. J. Food Microbiol. 1991, 13, 329–333. [Google Scholar] [CrossRef]
- Mylona, K.; Sulyok, M.; Magan, N. Fusarium graminearum and Fusarium verticillioides colonisation of wheat and maize, environmental factors, dry matter losses and mycotoxin production relevant to the EU legislative limits. Food. Add. Contam. 2012, 29, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Malachova, A.; Sulyok, M.; Beltran, E.; Berthiller, F.; Krska, R. Multi-Toxin Determination in Food—The Power of Dilute and Shoot Approaches in LC–MS–MS. LCGC Eur. 2015, 28, 542–555. [Google Scholar]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting environmental stress factors affects targetted metabolomic profiles in stored natural wheat and that inoculated with F. graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed]



| Natural Wheat | Natural Wheat + F. graminearum | Irradiated Grain + F. graminearum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | aw | ZEN1 | alpha-ZOL2 | beta-ZOL3 | ZEN1 | alpha-ZOL2 | beta-ZOL3 | ZEN1 | alpha-ZOL2 | beta-ZOL3 |
| 10 | 0.90 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 10 | 0.93 | 6.0 | <LOD | <LOD | 25.5 | <LOD | <LOD | 1.1 | <LOD | <LOD |
| 10 | 0.95 | <LOD | <LOD | <LOD | 1.0 | <LOD | <LOD | 0.9 | <LOD | <LOD |
| 15 | 0.90 | 16.7 | <LOD | <LOD | 195.9 | 2.6 | 12.0 | 16.7 | <LOD | 3.3 |
| 15 | 0.93 | 18.5 | 1.1 | 3.6 | 551.8 | 2.7 | 7.4 | 15.1 | <LOD | 3.3 |
| 15 | 0.95 | 131.9 | 1.2 | 6.7 | 241.9 | 2.9 | 8.2 | 22.1 | <LOD | 6.3 |
| 20 | 0.90 | 0.5 | <LOD | <LOD | 110.9 | <LOD | 3.2 | 120.8 | 2.2 | 24.6 |
| 20 | 0.93 | 1.0 | <LOD | <LOD | 11.4 | <LOD | <LOD | 178.4 | 2.5 | 43.0 |
| 20 | 0.95 | 777.5 | 13.3 | 51.9 | 810.2 | 9.1 | 35.7 | 219.8 | 4.9 | 78.7 |
| 25 | 0.90 | 112.5 | 2.8 | 16.3 | 382.6 | 8.5 | 16.4 | 1608.7 | 13.2 | 246.6 |
| 25 | 0.93 | 1536.9 | 19.9 | 84.9 | 1489.7 | 13.3 | 81.5 | 633.7 | 6.2 | 83.7 |
| 25 | 0.95 | 1167.8 | 15.6 | 59.7 | 1461.4 | 11.4 | 78.3 | 1030.2 | 10.2 | 227.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Cela, E.; Kiaitsi, E.; Sulyok, M.; Medina, A.; Magan, N. Fusarium graminearum in Stored Wheat: Use of CO2 Production to Quantify Dry Matter Losses and Relate This to Relative Risks of Zearalenone Contamination under Interacting Environmental Conditions. Toxins 2018, 10, 86. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020086
Garcia-Cela E, Kiaitsi E, Sulyok M, Medina A, Magan N. Fusarium graminearum in Stored Wheat: Use of CO2 Production to Quantify Dry Matter Losses and Relate This to Relative Risks of Zearalenone Contamination under Interacting Environmental Conditions. Toxins. 2018; 10(2):86. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020086
Chicago/Turabian StyleGarcia-Cela, Esther, Elsa Kiaitsi, Michael Sulyok, Angel Medina, and Naresh Magan. 2018. "Fusarium graminearum in Stored Wheat: Use of CO2 Production to Quantify Dry Matter Losses and Relate This to Relative Risks of Zearalenone Contamination under Interacting Environmental Conditions" Toxins 10, no. 2: 86. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10020086





