Development of an Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-Diacetoxyscirpenol and their Occurrence in Japanese Retail Food
Abstract
:1. Introduction
2. Results
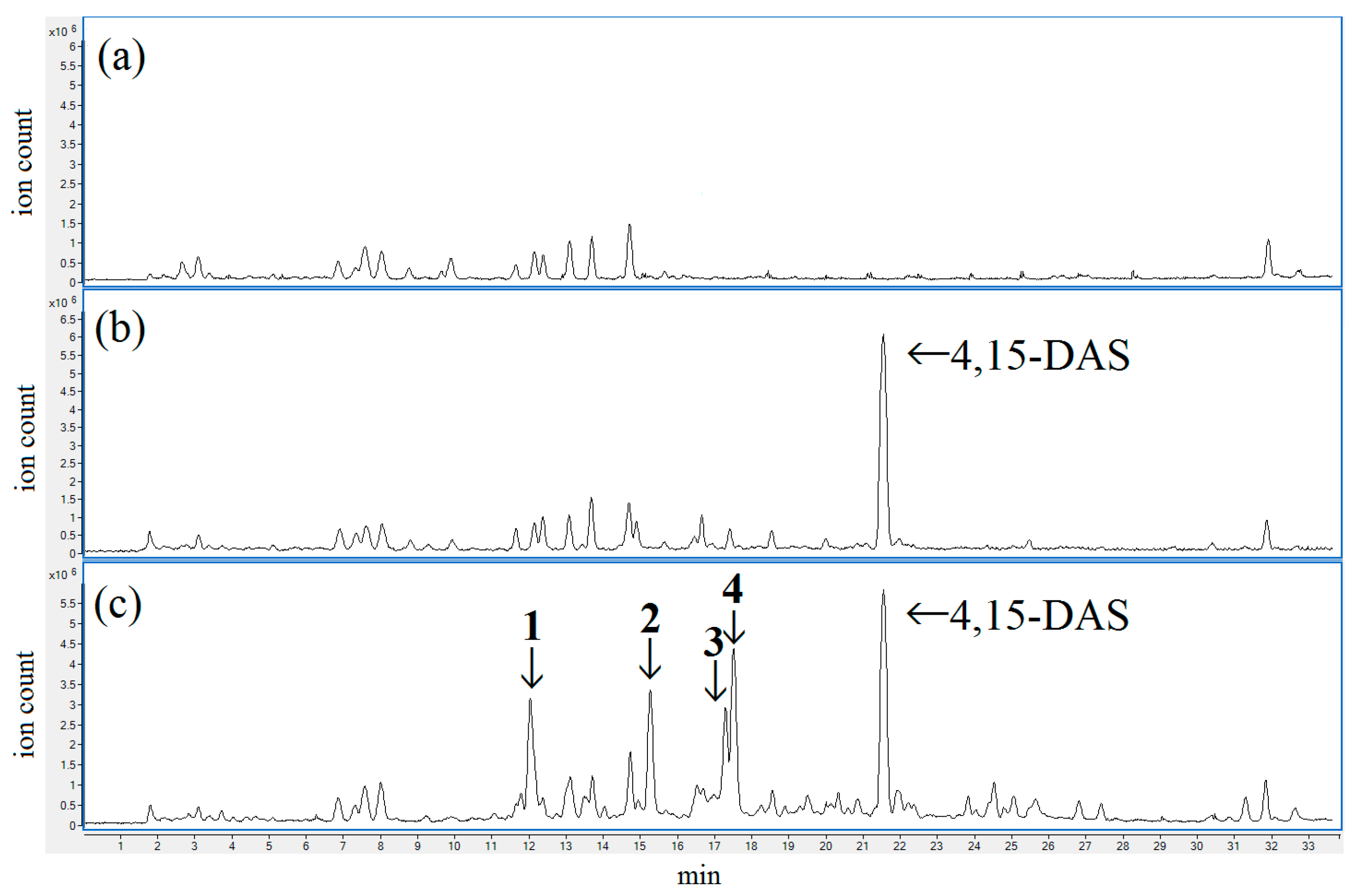
2.1. Screening of Fusarium Strains Producing Modified Forms of 4,15-DAS
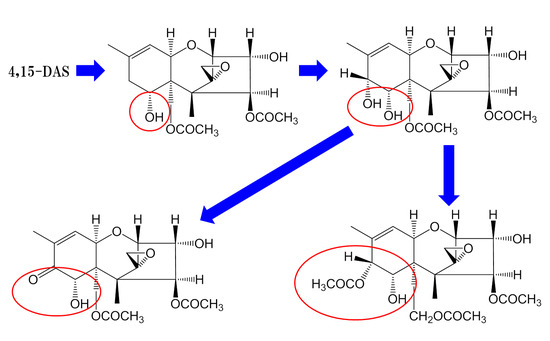
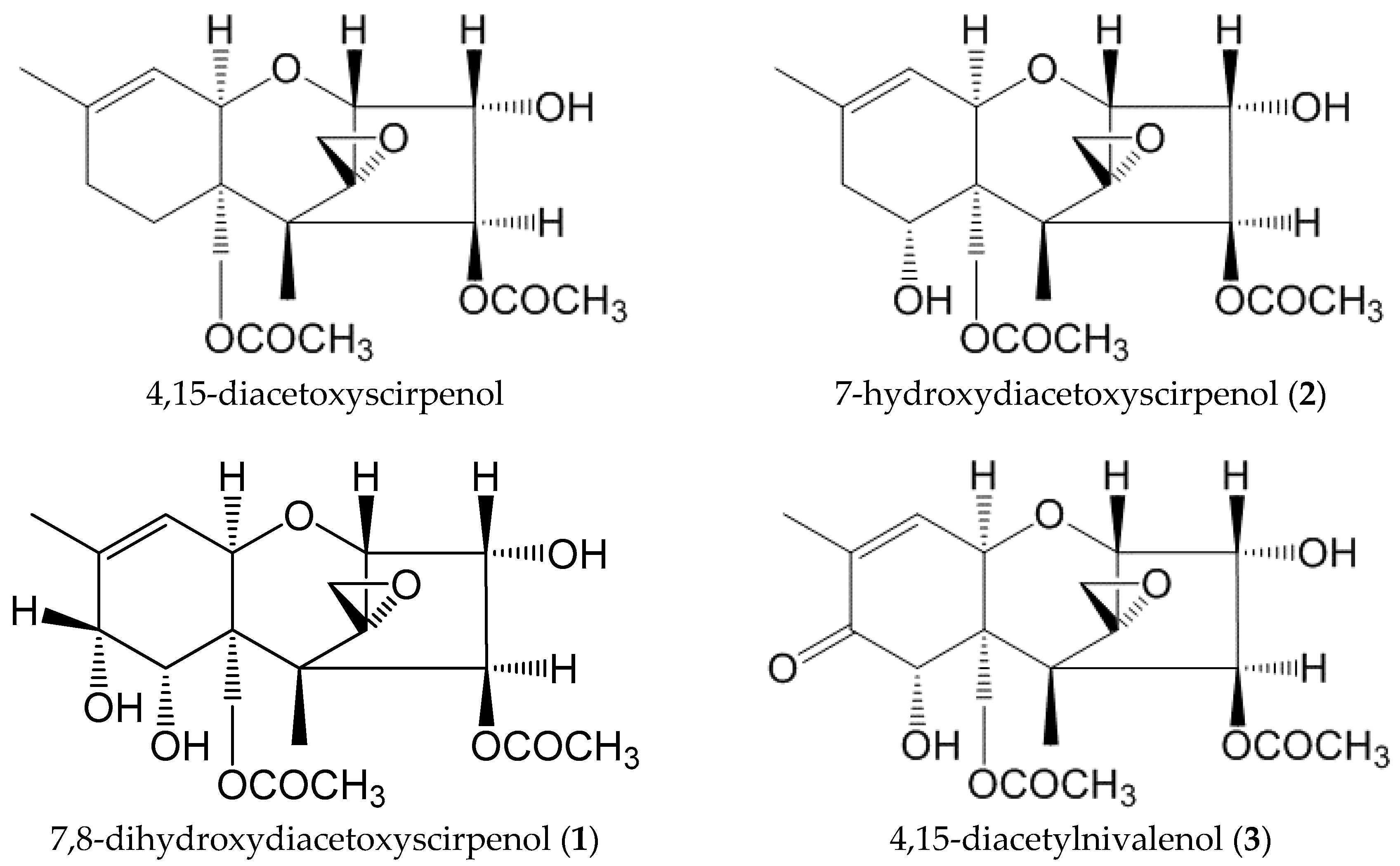
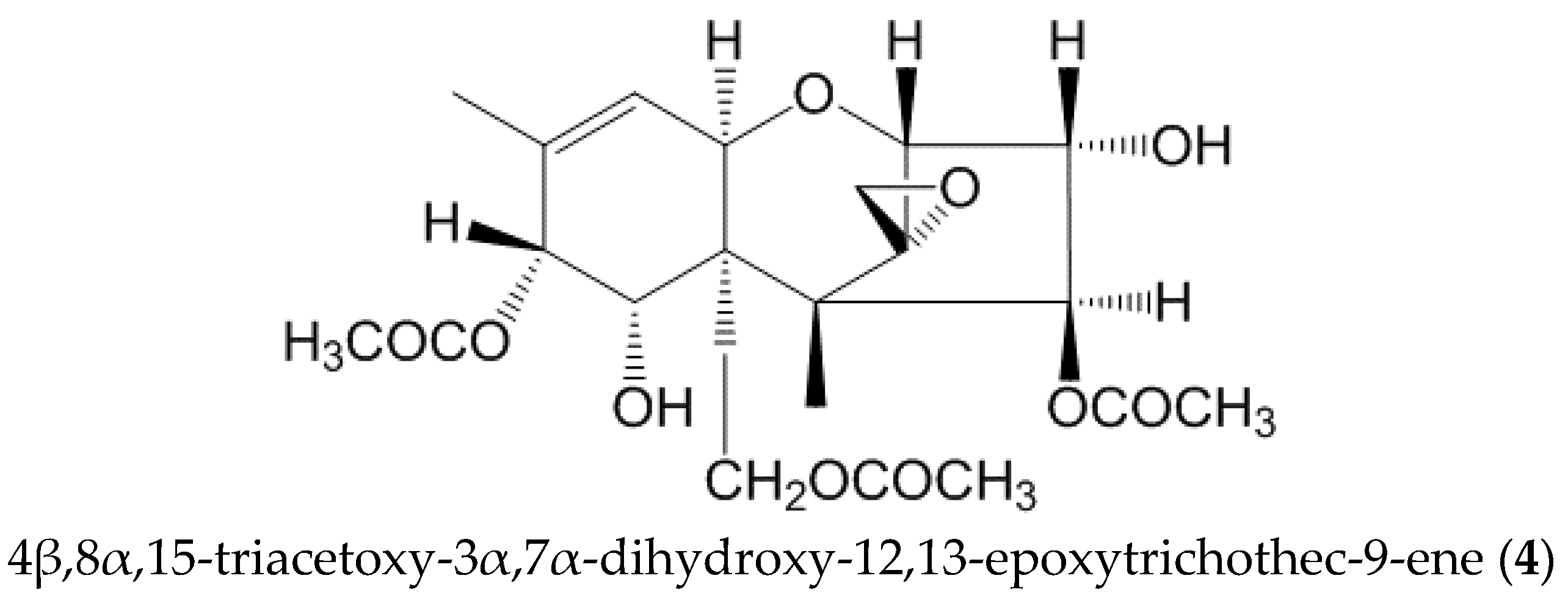
2.2. Identification of the Modified Forms of 4,15-DAS
2.3. Analytical Methods for Determination of 4,15-DAS and its Modified Forms
2.4. Occurrence of 4,15-DAS and its Modified Forms in Cereals
3. Discussion
4. Materials and Methods
4.1. Chemicals, Samples, and Strains
4.2. Sample Preparation for Q-TOF LC/MS Analysis
4.3. Q-TOF LC-MS Analysis Conditions
4.4. Purification of the Modified Forms of 4,15-DAS
4.5. Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-DAS
4.6. LC-MS/MS Analysis Conditions
4.7. Performance Evaluation of the Analytical Method
4.8. Calibration Curve
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Takeuchi, H.; Aoyama, K.; Taniguchi, M.; Hashiguchi, S.; Kai, S.; Ogiso, M.; Sato, T.; Akiyama, Y.; Nakajima, M.; et al. Occurrence of four Fusarium mycotoxins, deoxynivalenol, zearalenone, T-2 toxin, and HT-2 toxin, in wheat, barley, and Japanese retail food. J. Food Prot. 2014, 77, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Barros, G.; Zanon, M.A.; Palazzini, J.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A 2012, 29, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U.; Adler, A.; Clasen, P.-E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bottex, C.; Martin, A.; Fontanges, R. Action of a mycotoxin (diacetoxyscirpenol) on the immune response of the mouse-interaction with an immunomodulator (OM-89). Immunopharmacol. Immunotoxicol. 1990, 12, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Robison, T.; Mirocha, C.; Reddy, K. Acute toxicity of 12, 13-epoxytrichothecenes in one-day-old broiler chicks. Appl. Environ. Microbiol. 1978, 35, 636–640. [Google Scholar] [PubMed]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional wheat. Food Addit. Contam. Part A 2009, 26, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Gaba, D.; Chan, J.M. Monitoring of Fusarium trichothecenes in Canadian cereal grain shipments from 2010 to 2012. J. Agric. Food. Chem. 2013, 61, 7412–7418. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluation of Certain Contaminants in Food; WHO Technical Report Series, No. 1002; WHO: Geneva, Switzerland, 2017; pp. 40–54. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratz, S.W. Do plant-bound masked mycotoxins contribute to toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, J.-J.; Yu, C.-C.; Lu, X.-H.; Jiang, H.-R.; Shao, B.; Li, F.-Q. Simultaneous determination of masked deoxynivalenol and some important type B trichothecenes in Chinese corn kernels and corn-based products by ultra-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food. Chem. 2012, 60, 11638–11646. [Google Scholar]
- Yoshinari, T.; Ohnishi, T.; Kadota, T.; Sugita-Konishi, Y. Development of a purification method for simultaneous determination of deoxynivalenol and its acetylated and glycosylated derivatives in corn grits and corn flour by liquid chromatography–tandem mass spectrometry. J. Food Prot. 2012, 75, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Sakuda, S.; Furihata, K.; Furusawa, H.; Ohnishi, T.; Sugita-Konishi, Y.; Ishizaki, N.; Terajima, J. Structural determination of a nivalenol glucoside and development of an analytical method for the simultaneous determination of nivalenol and deoxynivalenol, and their glucosides, in wheat. J. Agric. Food. Chem. 2014, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Ajandouz, E.H.; Berdah, S.; Moutardier, V.; Bege, T.; Birnbaum, D.J.; Perrier, J.; Di Pasquale, E.; Maresca, M. Hydrolytic fate of 3/15-acetyldeoxynivalenol in humans: Specific deacetylation by the small intestine and liver revealed using In Vitro and Ex Vivo approaches. Toxins 2016, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.-D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- Grove, J.F. Phytotoxic compounds produced by Fusarium equiseti. Part VI. 4β,8α, 15-Triacetoxy-12,13-epoxytrichothec-9-ene-3α,7α-diol. J. Chem. Soc. C Org. 1970, 2, 378–379. [Google Scholar] [CrossRef]
- Ishii, K. Two new trichothecenes produced by Fusarium sp. Phytochemistry 1975, 14, 2469–2471. [Google Scholar] [CrossRef]
- Nakagawa, H.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H. Detection of masked mycotoxins derived from type A trichothecenes in corn by high-resolution LC-Orbitrap mass spectrometer. Food Addit. Contam. Part A 2013, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Morita, Y.; Tatsuno, T. Recherches toxicologiques sur les substances toxiques de Fusarium nivale: Etude chimique des toxins principales, nivalenol, fusarenon-X et nivalenol-4, 15-di-O-acetate. Chem. Pharm. Bull. 1972, 20, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.; Harris, L.; Alexander, N.; Ouellet, T.; Saparno, A.; Allard, S.; Desjardins, A. Tri1 in Fusarium graminearum encodes a P450 oxygenase. Appl. Environ. Microbiol. 2004, 70, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Peplow, A.W.; Meek, I.B.; Wiles, M.C.; Phillips, T.D.; Beremand, M.N. Tri16 is required for esterification of position C-8 during trichothecene mycotoxin production by Fusarium sporotrichioides. Appl. Environ. Microbiol. 2003, 69, 5935–5940. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Sato, N.; Ishii, K.; Sakai, K.; Tsunoda, H.; Enomoto, M. Biological and chemical detection of trichothecene mycotoxins of Fusarium species. Appl. Microbiol. 1973, 25, 699–704. [Google Scholar] [PubMed]
- Ueno, Y.; Ishii, K.; Sawano, M.; Ohtsubo, K.; Matsuda, Y. Toxicological approaches to the metabolites of Fusaria. XI. Trichothecenes and zearalenone from Fusarium species isolated from river sediments. Jpn. J. Exp. Med. 1977, 47, 177–184. [Google Scholar] [PubMed]

| Commodity | Conc. in Spiked Sample (μg/kg) | Recovery (%) a | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4,15-DAS | 7-HDAS | NES | 7,8-diHDAS | 4,15-diANIV | compound 4 | T-2 Toxin | HT-2 Toxin | ||||||||||||||||||
| Wheat flour | 5 | 98 | ± | 2 | 94 | ± | 9 | 101 | ± | 6 | 100 | ± | 5 | 103 | ± | 5 | 99 | ± | 5 | 91 | ± | 4 | 97 | ± | 4 |
| 50 | 100 | ± | 6 | 97 | ± | 8 | 98 | ± | 7 | 99 | ± | 8 | 93 | ± | 8 | 96 | ± | 7 | 92 | ± | 6 | 97 | ± | 5 | |
| Job’s tears products | 5 | 94 | ± | 5 | 94 | ± | 6 | 93 | ± | 6 | 96 | ± | 4 | 91 | ± | 8 | 91 | ± | 5 | 101 | ± | 3 | 98 | ± | 7 |
| 50 | 93 | ± | 5 | 99 | ± | 3 | 98 | ± | 6 | 99 | ± | 7 | 96 | ± | 6 | 97 | ± | 5 | 94 | ± | 6 | 95 | ± | 7 | |
| Rye flour | 5 | 97 | ± | 3 | 81 | ± | 6 | 100 | ± | 4 | 88 | ± | 2 | 94 | ± | 3 | 89 | ± | 3 | 96 | ± | 2 | 103 | ± | 4 |
| 50 | 100 | ± | 2 | 89 | ± | 6 | 100 | ± | 3 | 93 | ± | 2 | 89 | ± | 3 | 92 | ± | 4 | 98 | ± | 1 | 100 | ± | 1 | |
| Corn flour | 5 | 105 | ± | 4 | 101 | ± | 8 | 105 | ± | 4 | 102 | ± | 4 | 102 | ± | 5 | 99 | ± | 5 | 105 | ± | 2 | 107 | ± | 3 |
| 50 | 95 | ± | 3 | 97 | ± | 2 | 101 | ± | 4 | 100 | ± | 2 | 95 | ± | 1 | 91 | ± | 3 | 95 | ± | 3 | 103 | ± | 3 | |
| Azuki bean | 5 | 101 | ± | 5 | 92 | ± | 8 | 101 | ± | 13 | 103 | ± | 14 | 99 | ± | 5 | 104 | ± | 7 | 91 | ± | 4 | 99 | ± | 4 |
| 50 | 91 | ± | 3 | 88 | ± | 6 | 93 | ± | 9 | 97 | ± | 9 | 90 | ± | 3 | 99 | ± | 3 | 88 | ± | 2 | 89 | ± | 1 | |
| Analyte | LOD/LOQ (µg/kg) | Commodity | |||||
|---|---|---|---|---|---|---|---|
| Wheat Flour | Job’s Tears Product | Rye Flour | Corn Flour | Azuki Bean | |||
| No. of Sample | 101 | 46 | 41 | 27 | 33 | ||
| 4,15-DAS | Positive rate (%) a | 0 | 63 | 0 | 15 | 9 | |
| 0.1/0.2 | Mean (µg/kg) | - | 13 | - | 0.07 | 0.02 | |
| Maximum (µg/kg) | - | 70 | - | 0.8 | 0.3 | ||
| 7-HDAS | Positive rate (%) | 0 | 13 | 0 | 0 | 0 | |
| 0.2/0.5 | Mean (µg/kg) | - | 0.1 | - | - | - | |
| Maximum (µg/kg) | - | 2 | - | - | - | ||
| NES | Positive rate (%) | 0 | 13 | 0 | 0 | 0 | |
| 0.2/0.5 | Mean (µg/kg) | - | 0.2 | - | - | 0 | |
| Maximum (µg/kg) | - | 3 | - | - | - | ||
| 7,8-diHDAS | Positive rate (%) | 0 | 63 | 0 | 7 | 0 | |
| 0.1/0.2 | Mean (µg/kg) | - | 2 | - | 0.07 | - | |
| Maximum (µg/kg) | - | 10 | - | 1 | - | ||
| 4,15-diANIV | Positive rate (%) | 0 | 50 | 0 | 0 | 0 | |
| 0.2/0.5 | Mean (µg/kg) | - | 2 | - | - | - | |
| Maximum (µg/kg) | - | 16 | - | - | - | ||
| compound 4 | Positive rate (%) | 0 | 41 | 0 | 7 | 6 | |
| 0.1/0.2 | Mean (µg/kg) | - | 0.5 | - | 0.02 | 0.03 | |
| Maximum (µg/kg) | - | 4 | - | 0.4 | 0.6 | ||
| T-2 toxin | Positive rate (%) | 9 | 41 | 56 | 22 | 36 | |
| 0.1/0.2 | Mean (µg/kg) | 0.04 | 2 | 0.5 | 0.1 | 0.9 | |
| Maximum (µg/kg) | 1 | 28 | 4 | 1 | 8 | ||
| HT-2 toxin | Positive rate (%) | 26 | 41 | 76 | 15 | 55 | |
| 0.1/0.4 | Mean (µg/kg) | 0.4 | 2 | 2 | 0.1 | 1 | |
| Maximum (µg/kg) | 4 | 14 | 18 | 1 | 6 | ||
| Analyte | Area | |||
|---|---|---|---|---|
| Japan | South-East Asia | Unknown | ||
| No. of Sample | 28 | 13 | 5 | |
| 4,15-DAS | Positive rate (%) a | 39 | 100 | 100 |
| Mean (µg/kg) | 0.9 | 25 | 48 | |
| 7-HDAS | Positive rate (%) | 4 | 23 | 40 |
| Mean (µg/kg) | 0.06 | 0.2 | 0.4 | |
| NES | Positive rate (%) | 21 | 0 | 0 |
| Mean (µg/kg) | 0.3 | - | - | |
| 7,8-diHDAS | Positive rate (%) | 39 | 100 | 100 |
| Mean (µg/kg) | 0.5 | 4 | 5 | |
| 4,15-diANIV | Positive rate (%) | 68 | 15 | 40 |
| Mean (µg/kg) | 2 | 0.1 | 0.9 | |
| compound 4 | Positive rate (%) | 4 | 100 | 100 |
| Mean (µg/kg) | 0.03 | 1 | 1 | |
| T-2 toxin | Positive rate (%) | 68 | 0 | 0 |
| Mean (µg/kg) | 4 | - | - | |
| HT-2 toxin | Positive rate (%) | 68 | 0 | 0 |
| Mean (µg/kg) | 3 | - | - | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshinari, T.; Takeda, N.; Watanabe, M.; Sugita-Konishi, Y. Development of an Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-Diacetoxyscirpenol and their Occurrence in Japanese Retail Food. Toxins 2018, 10, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10050178
Yoshinari T, Takeda N, Watanabe M, Sugita-Konishi Y. Development of an Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-Diacetoxyscirpenol and their Occurrence in Japanese Retail Food. Toxins. 2018; 10(5):178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10050178
Chicago/Turabian StyleYoshinari, Tomoya, Nanami Takeda, Maiko Watanabe, and Yoshiko Sugita-Konishi. 2018. "Development of an Analytical Method for Simultaneous Determination of the Modified Forms of 4,15-Diacetoxyscirpenol and their Occurrence in Japanese Retail Food" Toxins 10, no. 5: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10050178