Arcanobacterium haemolyticum Phospholipase D Enzymatic Activity Promotes the Hemolytic Activity of the Cholesterol-Dependent Cytolysin Arcanolysin
Abstract
:1. Introduction
2. Results
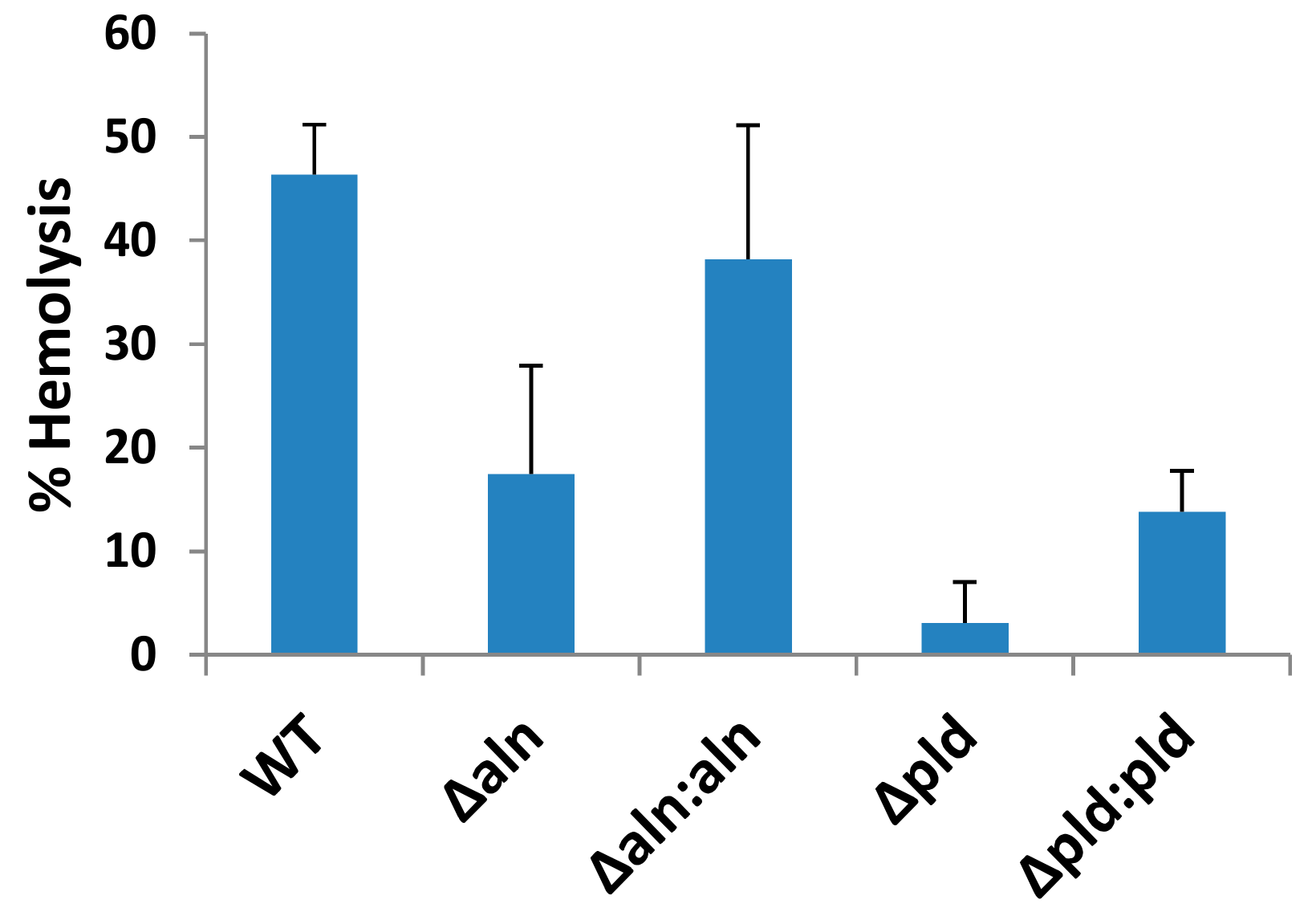
2.1. A. haemolyticum Produces at Least Two Products Which Contribute to Hemolytic Activity of Bacterial Supernatants
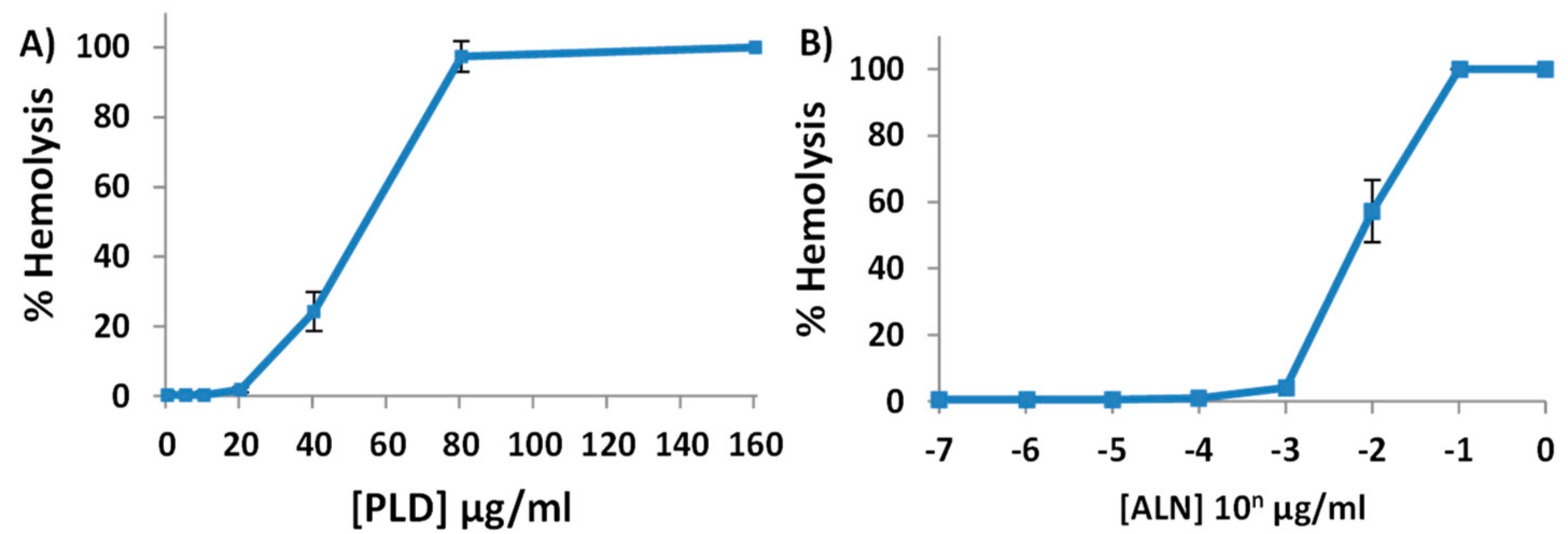
2.2. Purified His-ALN or His-PLD Is Sufficient to Cause Hemolysis of Human Erythrocytes
2.3. A. haemolyticum PLD Promotes ALN-Mediated Hemolysis in Both Time- and Concentration-Dependent Manners
2.4. An Increase in ALN-Mediated Hemolysis Is Not Dependent upon the Generation of Cyclic-Ceramide Phosphate
2.5. Treatment of Human Erythrocytes with PLD Subsequently Promotes ALN-Membrane Binding
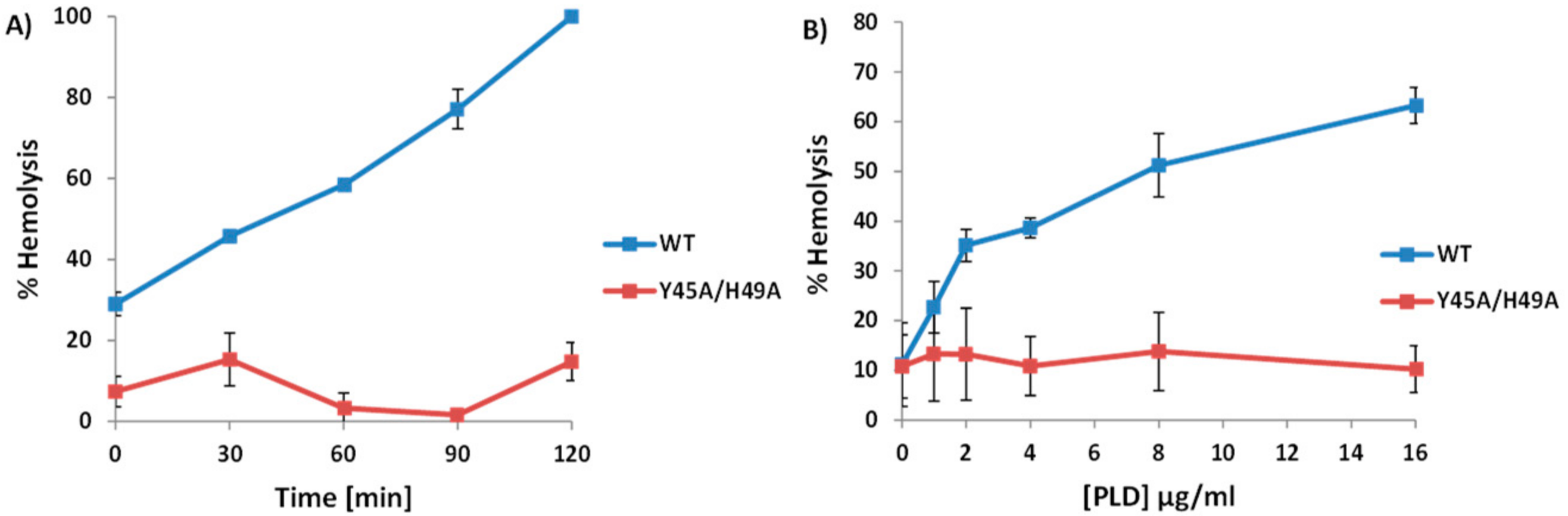
2.6. Individual Point Mutations within PLD Decreases Sphingomyelinase Activity
2.7. Sphingomyelinase-Deficient PLD Fails to Promote ALN-Mediated Hemolysis to the Same Extent as WT-PLD
2.8. The PLD-ALN Synergistic Relationship Also Exists in a Physiologically-Relevant Cell Line
3. Discussion
4. Materials and Methods
4.1. Bacteria and Growth Conditions
4.2. Construction of A. haemolyticum Mutants and Complementing Plasmids
4.3. DNA Techniques and Sequence Analysis
4.4. Purification of Recombinant His-PLD, His-ALN, His-SPH, and His-RocF
4.5. Hemolytic Assays
4.6. ALN-Binding Assays
4.7. Sphingomyelinase Assays
4.8. Mutagenesis of Pld or Aln
4.9. Cell Viability Assays
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Linder, R. Rhodococcus equi and Arcanobacterium haemolyticum: Two “coryneform” bacteria increasingly recognized as agents of human infection. Emerg. Infect. Dis. 1997, 3, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Banck, G.; Nyman, M. Tonsillitis and rash associated with Corynebacterium haemolyticum. J. Infect. Dis. 1986, 154, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.; Fuite, L.A.; Chan, F.T.; King, J.; Allen, U.; MacDonald, N.; Diaz-Mitoma, F. Incidence and pathogenicity of Arcanobacterium haemolyticum during a 2-year study in Ottawa. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1995, 21, 177–181. [Google Scholar] [CrossRef]
- Miller, R.A.; Brancato, F.; Holmes, K.K. Corynebacterium hemolyticum as a cause of pharyngitis and scarlatiniform rash in young adults. Ann. Intern. Med. 1986, 105, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Lucas, E.A.; Billington, S.J.; Ratner, A.J.; McGee, D.J. Arcanolysin is a cholesterol-dependent cytolysin of the human pathogen Arcanobacterium haemolyticum. BMC Microbiol. 2011, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R.K.; Hotze, E.M.; Wade, K.R. The unique molecular choreography of giant pore formation by the cholesterol-dependent cytolysins of Gram-Positive bacteria. Annu. Rev. Microbiol. 2015, 69, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R.K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.A.; Billington, S.J.; Carlson, P.; McGee, D.J.; Jost, B.H. Phospholipase D promotes Arcanobacterium haemolyticum adhesion via lipid raft remodeling and host cell death following bacterial invasion. BMC Microbiol. 2010, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, functionality, and chemical modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, D.M.; Cordes, M.H.J. Spider, bacterial and fungal phospholipase D toxins make cyclic phosphate products. Toxicon Off. J. Int. Soc. Toxinol. 2015, 108, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Brown, M.S.; Anderson, D.D.; Goldstein, J.L.; Radhakrishnan, A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 2014, 3, e02882. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.J.; Zabaleta, J.; Viator, R.J.; Testerman, T.L.; Ochoa, A.C.; Mendz, G.L. Purification and characterization of Helicobacter pylori arginase, RocF: Unique features among the arginase superfamily. Eur. J. Biochem. 2004, 271, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- García-Arribas, A.B.; Alonso, A.; Goñi, F.M. Cholesterol interactions with ceramide and sphingomyelin. Chem. Phys. Lipids 2016, 199, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pomerantsev, A.P.; Kalnin, K.V.; Osorio, M.; Leppla, S.H. Phosphatidylcholine-specific phospholipase C and sphingomyelinase activities in bacteria of the Bacillus cereus group. Infect. Immun. 2003, 71, 6591–6606. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.J.; Farrand, A.J.; Tweten, R.K. The cholesterol-dependent cytolysin signature motif: A critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 2012, 8, e1002787. [Google Scholar] [CrossRef]
- Farrand, A.J.; LaChapelle, S.; Hotze, E.M.; Johnson, A.E.; Tweten, R.K. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl. Acad. Sci. USA 2010, 107, 4341–4346. [Google Scholar] [CrossRef] [PubMed]
- Maclean, P.D.; Liebow, A.A.; Rosenberg, A.A. A hemolytic corynebacterium resembling Corynebacterium ovis and Corynebacterium pyogenes in man. J. Infect. Dis. 1946, 79, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Zaas, D.W.; Duncan, M.; Rae Wright, J.; Abraham, S.N. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta 2005, 1746, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Corrotte, M.; Fernandes, M.C.; Tam, C.; Andrews, N.W. Toxin Pores Endocytosed during Plasma Membrane Repair Traffic into the Lumen of MVBs for Degradation: Endocytosed SLO Pores are Degraded in Lysosomes. Traffic 2012, 13, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, A. Are penicillin treatment failures in Arcanobacterium haemolyticum pharyngotonsillitis caused by intracellularly residing bacteria? Scand. J. Infect. Dis. 1995, 27, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Hullin-Matsuda, F.; Taguchi, T.; Greimel, P.; Kobayashi, T. Lipid compartmentalization in the endosome system. Semin. Cell Dev. Biol. 2014, 31, 48–56. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.K.; Melville, S.B. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 2004, 72, 5204–5215. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, D.; Domann, E.; Buck, C.; Hain, T.; Hof, H.; Chakraborty, T.; Deckert-Schlüter, M. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 1998, 66, 5930–5938. [Google Scholar] [PubMed]
- Moe, P.C.; Heuck, A.P. Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers. Biochemistry (Mosc.) 2010, 49, 9498–9507. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, S.; Wessels, K.; Verdurmen, W.P.R.; Tomelleri, C.; Cluitmans, J.C.A.; Fransen, J.; Fuchs, B.; Schiller, J.; Joosten, I.; Brock, R.; et al. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis. 2012, 3, e410. [Google Scholar] [CrossRef] [PubMed]
- Langford, M.L.; Zabaleta, J.; Ochoa, A.C.; Testerman, T.L.; McGee, D.J. In vitro and in vivo complementation of the Helicobacter pylori arginase mutant using an intergenic chromosomal site. Helicobacter 2006, 11, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.J.; Hodgson, A.L. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid 1991, 25, 149–153. [Google Scholar] [CrossRef]
- Messerotti, L.J.; Radford, A.J.; Hodgson, A.L. Nucleotide sequence of the replication region from the Mycobacterium-Escherichia coli shuttle vector pEP2. Gene 1990, 96, 147–148. [Google Scholar] [CrossRef]
- Gekara, N.O.; Jacobs, T.; Chakraborty, T.; Weiss, S. The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization: Aggregation of lipid rafts by listeriolysin O. Cell. Microbiol. 2005, 7, 1345–1356. [Google Scholar] [CrossRef] [PubMed]




| Primer Name | Sequence (5′-3′) |
|---|---|
| DM1117 (T557A_Forward) | CGCGAGATCATCCTTCGCGGTACTGCCTTACGGCCTTGGTTC |
| DM1118 (T557A_Reverse) | GAACCAAGGCCGTAAGGCAGTACCGCGAAGGATGATCTCGCG |
| DM1121 (L558A_Forward) | CGCGAGATCATCCTTCGCGGTACTACCGCACGGCCTTGGTTC |
| DM1122 (L558A_Reverse) | GAACCAAGGCCGTGCGGTAGTACCGCGAAGGATGATCTCGCG |
| DM1125 (TL557_58AA_Forward) | CGCGAGATCATCCTTCGCGGTACTGCGGCACGGCCTTGGTTC |
| DM1126 (TL557_58AA_Reverse) | GAACCAAGGCCGTGCCGCAGTACCGCGAAGGATGATCTCGCG |
| DM1162 (P43A_Forward) | ACCAACCACTGGTAACCGTGCCGTCTATGCCATTGCGCACC |
| DM1163 (P43A_Reverse) | GGTGCGCAATGGCATAGACGGCACGGTTACCAGTGGTTGGT |
| DM1164 (Y45A_Forward) | AGAACACGGTGCGCAATGGCGGCGACTGGACGGTTACCAGTGG |
| DM1165 (Y45A_Reverse) | CCACTGGTAACCGTCCAGTCGCCGCCATTGCGCACCGTGTTCT |
| DM1166(H49A_Forward) | TTTGCTTCGTCAGAACACGGGCCGCAATGGCATAGACTGGAC |
| DM1167(H49A_Reverse) | GTCCAGTCTATGCCATTGCGGCCCGTGTTCTGACGAAGCAAA |
| DM1168(N68A_Forward) | AGTAAAATCAATTTCCAGAGCGGCCGCGCCAATTTTGATTGCGTC |
| DM1169(N68A_Reverse) | GACGCAATCAAAATTGGCGCGGCCGCTCTGGAAATTGATTTTACT |
| DM1170(H272A_Forward) | AATGTCTTTGTTGGTTGCCATGGCGTGAGTACCTTGATGTGCATC |
| DM1171(H272A_Reverse) | GGATGCACATCAAGGTACTCACGCCATGGCAACCAACAAAGACAT |
| DM1172(T275A_Forward) | ACGGAATGTCTTTGTTGGCTGCCATGTGGTGAGTACCTTG |
| DM1173(T275A_Reverse) | CAAGGTACTCACCACATGGCAGCCAACAAAGACATTCCGT |
| DM1174 (P43A,Y45A_F) | CGGTGCGCAATGGCGGCGACGGCACGGTTACCAGTGGTTGGTTGTTCTT |
| DM1175 (P43A,Y45A_R) | AAGAACAACCAACCACTGGTAACCGTGCCGTCGCCGCCATTGCGCACCG |
| DM1176 (P43A,H49A_F) | CTTTGCTTCGTCAGAACACGGGCCGCAATGGCATAGACGGCACGG |
| DM1177 (P43A,H49A_R) | CCGTGCCGTCTATGCCATTGCGGCCCGTGTTCTGACGAAGCAAAG |
| DM1178 (Y45A,H49A_F) | GCTTCGTCAGAACACGGGCCGCAATGGCGGCGACTGGACGGTTACCAG |
| DM1179 (Y45A,H49A_R) | CTGGTAACCGTCCAGTCGCCGCCATTGCGGCCCGTGTTCTGACGAAGC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellings, P.S.; McGee, D.J. Arcanobacterium haemolyticum Phospholipase D Enzymatic Activity Promotes the Hemolytic Activity of the Cholesterol-Dependent Cytolysin Arcanolysin. Toxins 2018, 10, 213. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10060213
Gellings PS, McGee DJ. Arcanobacterium haemolyticum Phospholipase D Enzymatic Activity Promotes the Hemolytic Activity of the Cholesterol-Dependent Cytolysin Arcanolysin. Toxins. 2018; 10(6):213. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10060213
Chicago/Turabian StyleGellings, Patrick S., and David J. McGee. 2018. "Arcanobacterium haemolyticum Phospholipase D Enzymatic Activity Promotes the Hemolytic Activity of the Cholesterol-Dependent Cytolysin Arcanolysin" Toxins 10, no. 6: 213. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10060213




