Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum
Abstract
:1. Introduction
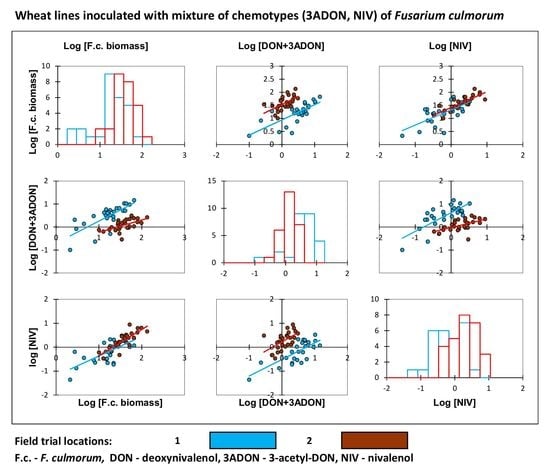
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fusarium culmorum Isolates
5.2. Field Experiments
5.3. DNA Extraction
5.4. Fusarium culmorum DNA Analysis
5.5. Analysis of Mycotoxins
5.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snijders, C.H.A. Resistance in wheat to Fusarium infection and trichothecene formation. Toxicol. Lett. 2004, 153, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.G.; Komoróczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Foroud, N.A.; Eudes, F. Trichothecenes in cereal grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Mesterházy, Á. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Argyris, J.; Van Sanford, D.; TeKrony, D. Fusarium graminearum infection during wheat seed development and its effect on seed quality. Crop Sci. 2003, 43, 1782–1788. [Google Scholar] [CrossRef]
- Horevaj, P.; Gale, L.; Milus, E. Resistance in winter wheat lines to initial infection and spread within spikes by deoxynivalenol and nivalenol chemotypes of Fusarium graminearum. Plant Dis. 2011, 95, 31–37. [Google Scholar] [CrossRef]
- Kubo, K.; Kawada, N.; Fujita, M.; Hatta, K.; Oda, S.; Nakajima, T. Effect of cleistogamy on Fusarium head blight resistance in wheat. Breed. Sci. 2010, 60, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Skinnes, H.; Semagn, K.; Tarkegne, Y.; Marøy, A.G.; Bjørnstad, Å. The inheritance of anther extrusion in hexaploid wheat and its relationship to Fusarium head blight resistance and deoxynivalenol content. Plant Breed. 2010, 129, 149–155. [Google Scholar] [CrossRef]
- Lu, Q.; Lillemo, M.; Skinnes, H.; He, X.; Shi, J.; Ji, F.; Dong, Y.; Bjørnstad, A. Anther extrusion and plant height are associated with Type I resistance to Fusarium head blight in bread wheat line “Shanghai-3/Catbird”. Theor. Appl. Genet. 2013, 126, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Kociuba, W.; Kramek, A. The analysis of some characteristics of triticale flowering biology suitable for breeding and reproduction of cultivars. Ann. UMCS 2004, 59, 115–122. (In Polish) [Google Scholar]
- Kubo, K.; Fujita, M.; Kawada, N.; Nakajima, T.; Nakamura, K.; Maejima, H.; Ushiyama, T.; Hatta, K.; Matsunaka, H. Minor differences in anther extrusion affect resistance to Fusarium head blight in wheat. J. Phytopathol. 2013, 161, 308–314. [Google Scholar] [CrossRef]
- Miller, S.S.; Chabot, D.M.P.; Ouellet, T.; Harris, L.J.; Fedak, G. Use of a Fusarium graminearum strain transformed with green fluorescent protein to study infection in wheat (Triticum aestivum). Can. J. Plant Pathol. 2004, 26, 453–463. [Google Scholar] [CrossRef]
- Strange, R.N.; Smith, H. A fungal growth stimulant in anthers, which predisposes wheat to attack by Fusarium graminearum. Physiol. Plant Pathol. 1971, 1, 141–150. [Google Scholar] [CrossRef]
- Strange, R.N.; Majer, J.R.; Smith, H. The isolation and identification of choline and betaine as the two major components in anthers and wheat germ that stimulate Fusarium graminearum in vitro. Physiol. Plant Pathol. 1974, 4, 277–290. [Google Scholar] [CrossRef]
- Pearce, R.B.; Strange, R.N.; Smith, H. Glycinebetaine and choline in wheat: Distribution and relation to infection by Fusarium graminearum. Phytochemistry 1976, 15, 953–954. [Google Scholar] [CrossRef]
- Kang, Z.; Buchenauer, H. Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum. Mycol. Res. 2000, 104, 1083–1093. [Google Scholar] [CrossRef]
- Rudd, J.C.; Horsley, R.D.; McKendry, A.L.; Elias, E.M. Host plant resistance genes for Fusarium head blight. Crop Sci. 2001, 41, 620. [Google Scholar] [CrossRef]
- Gosman, N.; Steed, A.; Chandler, E.; Thomsett, M.; Nicholson, P. Evaluation of type I Fusarium head blight resistance of wheat using non-deoxynivalenol-producing fungi. Plant Pathol. 2010, 59, 147–157. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Buerstmayr, M.; Schweiger, W.; Steiner, B. Breeding for resistance to head blight caused by Fusarium spp. in wheat. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2014, 9, 236–276. [Google Scholar] [CrossRef]
- Bai, G.H.; Shaner, G.; Ohm, H. Inheritance of resistance to Fusarium graminearum in wheat. Theor. Appl. Genet. 2000, 100, 1–8. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Lemmens, M.; Hartl, L.; Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (type II resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Rudd, R.; Rudd, J. A point inoculation method for evaluating scab resistance in wheat. In 1999 National Fusarium Head Blight Forum; Wagester, J.A., Ward, R., Hart, L.P., Hazen, S.P., Lewis, J., Borden, H., Eds.; Michigan State University: East Lansing, MI, USA, 1999; p. 128. [Google Scholar]
- Bai, G.H.; Plattner, R.; Desjardins, A.; Kolb, F. Resistance to Fusarium head blight and deoxynivalenol accumulation in wheat. Plant Breed. 2001, 120, 1–6. [Google Scholar] [CrossRef]
- Argyris, J.; TeKrony, D.; Hershman, D.; VanSanford, D.; Hall, M.; Kennedy, B.; Rucker, M.; Edge, C. Fusarium head blight infection following point inoculation in the greenhouse compared with movement of Fusarium graminearum in seed and floral components. Crop Sci. 2005, 45, 626–634. [Google Scholar] [CrossRef]
- Miedaner, T.; Moldovan, M.; Ittu, M. Comparison of spray and point inoculation to assess resistance to Fusarium head blight in a multienvironment wheat trial. Phytopathology 2003, 93, 1068–1072. [Google Scholar] [CrossRef]
- Van Ginkel, M.; Gilchrist, L. How to make intelligent crosses to accumulate Fusarium head blight resistance genes based on knowledge of the underlying resistance mechanisms. In 2002 National Fusarium Head Blight Forum Proceedings; Canty, S., Lewis, J., Siler, L., Ward, R.W., Eds.; Michigan State University: East Lansing, MI, USA, 2002; pp. 268–272. [Google Scholar]
- Bai, G.; Kolb, F.L.; Shaner, G.; Domier, L.L. Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 1999, 89, 343–348. [Google Scholar] [CrossRef]
- Zwart, R.S.; Muylle, H.; Van Bockstaele, E.; Roldán-Ruiz, I. Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor. Appl. Genet. 2008, 117, 813. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Buerstmayr, H.; Krska, R.; Schuhmacher, R.; Grausgruber, H.; Ruckenbauer, P. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur. J. Plant Pathol. 2004, 110, 299–308. [Google Scholar] [CrossRef]
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Doohan, F.M.; Parry, D.W.; Nicholson, P. Fusarium ear blight of wheat: The use of quantitative PCR and visual disease assessment in studies of disease control. Plant Pathol. 1999, 48, 209–217. [Google Scholar] [CrossRef]
- Schnerr, H.; Vogel, R.F.; Niessen, L. Correlation between DNA of trichothecene-producing Fusarium species and deoxynivalenol concentrations in wheat-samples. Lett. Appl. Microbiol. 2002, 35, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Van Maanen, A.; Moretti, A.; et al. Is the amount of mycotoxins in cereal grains related to the quantity of Fusarium DNA? Asp. Appl. Biol. 2003, 68, 101–108. [Google Scholar]
- Opoku, N.; Back, M.A.; Edwards, S.G. Susceptibility of cereal species to Fusarium langsethiae under identical field conditions. Eur. J. Plant Pathol. 2018, 150, 869–879. [Google Scholar] [CrossRef]
- Leišová, L.; Kučera, L.; Chrpová, J.; Sýkorová, S.; Šíp, V.; Ovesná, J. Quantification of Fusarium culmorum in wheat and barley tissues using real-time PCR in comparison with DON content. J. Phytopathol. 2006, 154, 603–611. [Google Scholar] [CrossRef]
- Waalwijk, C.; Van Der Heide, R.; De Vries, I.; Van Der Lee, T.; Schoen, C.; Costrel-de Corainville, G.; Häuser-Hahn, I.; Kastelein, P.; Köhl, J.; Lonnet, P.; et al. Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 2004, 110, 481–494. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- Reischer, G.H.; Lemmens, M.; Farnleitner, A.; Adler, A.; Mach, R.L. Quantification of Fusarium graminearum in infected wheat by species specific real-time PCR applying a TaqMan probe. J. Microbiol. Methods 2004, 59, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, E.; Zgoła-Grześkowiak, A.; Waśkiewicz, A.; Stępień, Ł.; Beszterda, M. Can ergosterol be an indicator of Fusarium fungi and mycotoxins in cereal products? J. Braz. Chem. Soc. 2015, 15, 18–23. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Von der Ohe, C.; Miedaner, T. Competitive aggressiveness in binary mixtures of Fusarium graminearum and F. culmorum isolates inoculated on spring wheat with highly effective resistance QTL. J. Phytopathol. 2010, 410. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jensen, J.D.; Rodríguez, A.; Jørgensen, L.N.; Justesen, A.F. TRI12 based quantitative real-time PCR assays reveal the distribution of trichothecene genotypes of F. graminearum and F. culmorum isolates in Danish small grain cereals. Int. J. Food Microbiol. 2012, 157, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Sun, H.Y.; Li, W.; Xia, Y.L.; Deng, Y.Y.; Zhang, A.X.; Chen, H.G. Fitness of three chemotypes of Fusarium graminearum species complex in major winter wheat-producing areas of China. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S.; Kistler, H.C. Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology 2005, 95, 1397–1404. [Google Scholar] [CrossRef]
- Lemmens, M.; Koutnik, A.; Steiner, B.; Buerstmayr, H.; Berthiller, F.; Schuhmacher, R.; Maier, F.; Schäfer, W. Investigations on the ability of Fhb1 to protect wheat against nivalenol and deoxynivalenol. Cereal Res. Commun. 2008, 36, 429–435. [Google Scholar] [CrossRef]
- Eudes, F.; Comeau, A.; Collin, S.R. Phytotoxicité de huit mycotoxines associées à la fusariose de l’épi chez le blé. Can. J. Plant Pathol. 2000, 22, 286–292. [Google Scholar] [CrossRef]
- Foroud, N.A.; McCormick, S.P.; MacMillan, T.; Badea, A.; Kendra, D.F.; Ellis, B.E.; Eudes, F. Greenhouse studies reveal increased aggressiveness of emergent Canadian Fusarium graminearum chemotypes in wheat. Plant Dis. 2012, 96, 1271–1279. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.M.; Chou, T.C.; Oswald, I.P. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Kovalsky Paris, M.P.; Paolino, G.; Bürstmayr, H.; Lemmens, M.; Berthiller, F.; Schuhmacher, R.; Krska, R.; MacH, R.L. A reference-gene-based quantitative PCR method as a tool to determine Fusarium resistance in wheat. Anal. Bioanal. Chem. 2009, 395, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Horevaj, P.; Milus, E.A.; Bluhm, B.H. A real-time qPCR assay to quantify Fusarium graminearum biomass in wheat kernels. J. Appl. Microbiol. 2011, 111, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Scheider, N.; Guo, J.R.; Verreet, J.A.; Beyer, M. Assessing the intensity of Fusarium-damage in wheat: A comparison of selected disease parameters during disease development and the role of fungicides. J. Plant Dis. Prot. 2009, 116, 118–123. [Google Scholar] [CrossRef]
- Schlang, N.; Duveiller, E. Current approaches and utilization of new screening techniques for evaluation of FHB resistance at CIMMYT. Plant Breed. Seed Sci. 2011, 64. [Google Scholar] [CrossRef]
- Rossi, V.; Terzi, V.; Moggi, F.; Morcia, C.; Faccioli, P.; Haidukowski, M.; Pascale, M. Assessment of Fusarium infection in wheat heads using a quantitative polymerase chain reaction (qPCR) assay. Food Addit. Contam. 2007, 24, 1121–1130. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Tóth, B.; Bartók, T.; Varga, M. Breeding strategies against FHB in winter wheat and their relation to type I resistance. Cereal Res. Commun. 2008, 36, 37–43. [Google Scholar] [CrossRef]
- Mesterházy, Á. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Miedaner, T.; Heinrich, N.; Schneider, B.; Oettler, G.; Rohde, S.; Rabenstein, F. Estimation of deoxynivalenol (DON) content by symptom rating and exoantigen content for resistance selection in wheat and triticale. Euphytica 2004, 139, 123–132. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Walentyn-Góral, D. Higher Fusarium toxin accumulation in grain of winter triticale lines inoculated with Fusarium culmorum as compared with wheat. Toxins 2016, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Schneider, B.; Oettler, G. Means and variances for Fusarium head blight resistance of F2-derived bulks from winter triticale and winter wheat crosses. Euphytica 2006, 152, 405–411. [Google Scholar] [CrossRef]
- Oettler, G.; Heinrich, N.; Miedaner, T. Estimates of additive and dominance effects for Fusarium head blight resistance of winter triticale. Plant Breed. 2004, 123, 525–530. [Google Scholar] [CrossRef]
- Oettler, G.; Wahle, G. Genotypic and environmental variation of resistance to head blight in triticale inoculated with Fusarium culmorum. Plant Breed. 2001, 120, 297–300. [Google Scholar] [CrossRef]
- Ochodzki, P.; Góral, T. Production of mycotoxins by selected Fusarium graminearum and F. culmorum isolates cultured on rice and wheat. In Proceedings of the Conference on 28 Mykotoxin-Workshop, Bydgoszcz, Poland, 29–31 May 2006; p. 73. [Google Scholar]
- Wiśniewska, H.; Kowalczyk, K. Resistance of cultivars and breeding lines of spring wheat to Fusarium culmorum and powdery mildew. J. Appl. Genet. 2005, 46, 35–40. [Google Scholar] [PubMed]
- Snijders, C.H.A.; Perkowski, J. Effects of head blight caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology 1990, 80, 566–570. [Google Scholar] [CrossRef]
- Chełkowski, J.; Gromadzka, K.; Stępień, Ł.; Lenc, L.; Kostecki, M.; Berthiller, F. Fusarium species, zearalenone and deoxynivalenol content in preharvest scabby wheat heads from Poland. World Mycotoxin J. 2012, 5, 133–141. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium species infecting wheat heads in Poland. Open Life Sci. 2014, 9, 163–172. [Google Scholar] [CrossRef]
- Stepień, L.; Gromadzka, K.; Chelkowski, J. Polymorphism of mycotoxin biosynthetic genes among Fusarium equiseti isolates from Italy and Poland. J. Appl. Genet. 2012, 53, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium head blight of cereals in Denmark: Species complex and related mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Weingaertner, J.; Krska, R.; Praznik, W.; Grasserbauer, M.; Lew, H. Use of Mycosep multifunctional clean-up columns for the determination of trichothecenes in wheat by electron-capture gas chromatography. Fresenius J. Anal. Chem. 1997, 357, 1206–1210. [Google Scholar] [CrossRef]



| No. | Line 1 | Fusarium Head Blight Index (%) | Fusarium Damaged Kernels (%) | F.c. Biomass (pg/ng) | DON 2 (mg/kg) | NIV (mg/kg) | TRT 3 (mg/kg) | Biomass/TRT Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | MHR 5 | 15.3 | 20.7 | 19.3 | 0.371 | 0.348 | 0.719 | 26.8 |
| 2 | MHR 2 | 6.9 | 9.4 | 7.3 | 1.057 | 0.482 | 1.539 | 4.7 |
| 3 | STH 1 | 24.1 | 24.0 | 21.4 | 0.608 | 1.001 | 1.609 | 13.3 |
| 4 | SMH 2 | 12.9 | 22.0 | 34.1 | 0.576 | 1.198 | 1.774 | 19.2 |
| 5 | STH 8 | 13.2 | 10.8 | 14.2 | 1.498 | 0.335 | 1.833 | 5.3 |
| 6 | DANKO 4 | 18.1 | 15.8 | 37.0 | 1.532 | 0.702 | 2.234 | 16.6 |
| 7 | MHR 1 | 11.1 | 24.1 | 25.9 | 1.428 | 0.939 | 2.367 | 10.9 |
| 8 | SMH 3 | 15.4 | 22.6 | 24.6 | 0.870 | 1.533 | 2.403 | 10.2 |
| 9 | STH 5 | 17.5 | 13.0 | 19.7 | 1.805 | 1.007 | 2.812 | 7.0 |
| 10 | PHR 2 | 17.8 | 15.4 | 23.0 | 2.100 | 1.148 | 3.248 | 7.1 |
| 11 | SMH 1 | 17.2 | 24.4 | 21.3 | 1.911 | 1.557 | 3.468 | 6.1 |
| 12 | STH 7 | 10.8 | 11.2 | 16.5 | 2.566 | 1.094 | 3.660 | 4.5 |
| 13 | DANKO 5 | 19.1 | 26.7 | 22.5 | 2.840 | 0.832 | 3.672 | 6.1 |
| 14 | SMH 4 | 22.4 | 33.6 | 36.6 | 2.673 | 1.383 | 4.056 | 9.0 |
| 15 | Tonacja cv. | 12.3 | 27.8 | 25.3 | 2.717 | 1.515 | 4.232 | 6.0 |
| 16 | PHR 3 | 13.2 | 26.3 | 24.5 | 3.005 | 1.667 | 4.672 | 7.7 |
| 17 | PHR 1 | 12.5 | 34.6 | 29.9 | 2.286 | 2.573 | 4.859 | 6.1 |
| 18 | MHR 3 | 23.5 | 30.3 | 33.8 | 3.096 | 1.795 | 4.891 | 6.9 |
| 19 | MHR 4 | 17.6 | 15.6 | 27.3 | 4.173 | 1.249 | 5.422 | 5.0 |
| 20 | DANKO 2 | 9.5 | 22.5 | 37.1 | 4.304 | 1.602 | 5.906 | 6.3 |
| 21 | DANKO 3 | 19.0 | 32.9 | 50.8 | 3.068 | 3.064 | 6.132 | 8.3 |
| 22 | STH 3 | 28.8 | 45.1 | 87.8 | 3.804 | 3.253 | 7.057 | 12.4 |
| 23 | STH 2 | 44.7 | 52.8 | 67.6 | 3.262 | 4.110 | 7.372 | 9.2 |
| 24 | PHR 4 | 16.0 | 18.0 | 33.5 | 6.005 | 1.540 | 7.545 | 4.4 |
| 25 | STH 4 | 24.6 | 57.8 | 66.6 | 5.811 | 3.612 | 9.423 | 7.1 |
| 26 | STH 6 | 30.6 | 42.8 | 50.7 | 6.742 | 3.534 | 10.276 | 4.9 |
| 27 | DANKO 1 | 22.0 | 50.1 | 59.6 | 8.303 | 4.998 | 13.301 | 4.5 |
| Mean | 18.4 | 27.0 | 34.0 | 2.904 | 1.780 | 4.684 | 8.7 |
| Class | No. of Lines | FHBi (%) | FDK (%) | F.c. Biomass (pg/ng) | DON 1 (mg/kg) | NIV (mg/kg) | Biomass/TRT 2 Ratio |
|---|---|---|---|---|---|---|---|
| 1 | 13 | 15.2 (6.9–24.1) | 17.5 (9.4–26.7) | 23.4 (7.3–37.1) | 2.330 (0.371–6.005) | 0.944 (0.335–1.602) | 9.3 (4.4–26.8) |
| 2 | 8 | 16.2 (12.3–23.5) | 27.7 (22.0–34.6) | 28.8 (21.3–36.6) | 2.142 (0.576–3.096) | 1.653 (1.198–2.573) | 8.6 (5.3–19.2) |
| 3 | 6 | 28.3 (19.0–44.7) | 46.9 (32.9–57.8) | 66.5 (50.7–87.8) | 5.165 (3.068–8.303) | 3.762 (3.064–4.998) | 7.7 (4.5–12.4) |
| Total/mean | 27 | 18.4 | 27.0 | 34.0 | 2.904 | 1.780 | 8.7 |
| Variables (n = 54) | FHBi (%) | FDK (%) | F.c. Biomass (pg/ng) | DON 1 (mg/kg) | NIV (mg/kg) | TRT (mg/kg) |
|---|---|---|---|---|---|---|
| FDK [%] | 0.780 | |||||
| F.c. biomass [pg/ng) | 0.648 | 0.792 | ||||
| DON [mg/kg) | −0.173 | 0.102 | 0.319 | |||
| NIV [mg/kg) | 0.672 | 0.802 | 0.801 | 0.291 | ||
| TRT [mg/kg) | 0.191 | 0.509 | 0.649 | 0.858 | 0.704 | |
| Biomass/TRT ratio | 0.545 | 0.339 | 0.421 | −0.641 | 0.117 | −0.418 |
| Variables | FHBi C | FHBi R | FDK C | FDK R | F.c. Biomass C | F.c. Biomass R | DON 1 C | DON 1 R | NIV C |
|---|---|---|---|---|---|---|---|---|---|
| FHBi R | 0.464 | ||||||||
| FDK C | 0.578 | 0.610 | |||||||
| FDK R | 0.146 | 0.670 | 0.491 | ||||||
| F.c. biomass C | 0.381 | 0.388 | 0.672 | 0.285 | |||||
| F.c. biomass R | 0.259 | 0.744 | 0.559 | 0.796 | 0.254 | ||||
| DON 1 C | 0.212 | 0.243 | 0.663 | 0.185 | 0.748 | 0.174 | |||
| DON 1 R | 0.102 | 0.514 | 0.523 | 0.462 | 0.374 | 0.580 | 0.482 | ||
| NIV C | 0.428 | 0.268 | 0.589 | 0.208 | 0.692 | 0.194 | 0.750 | 0.509 | |
| NIV R | 0.125 | 0.711 | 0.484 | 0.899 | 0.328 | 0.813 | 0.383 | 0.540 | 0.280 |
| Target | Primer Name | Sequence (5′–3′) |
|---|---|---|
| F. culmorum | FculC561 fwd FculC614 rev | CACCGTCATTGGTATGTTGTCACT CGGGAGCGTCTGATAGTCG |
| Plant TEF-1α | Hor1f Hor2r | TCTCTGGGTTTGAGGGTGAC GGCCCTTGTACCAGTCAAGGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum. Toxins 2019, 11, 2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010002
Góral T, Wiśniewska H, Ochodzki P, Nielsen LK, Walentyn-Góral D, Stępień Ł. Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum. Toxins. 2019; 11(1):2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010002
Chicago/Turabian StyleGóral, Tomasz, Halina Wiśniewska, Piotr Ochodzki, Linda Kærgaard Nielsen, Dorota Walentyn-Góral, and Łukasz Stępień. 2019. "Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum" Toxins 11, no. 1: 2. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010002