Bioactivity and Structural Properties of Novel Synthetic Analogues of the Protozoan Toxin Climacostol
Abstract
:1. Introduction
2. Results
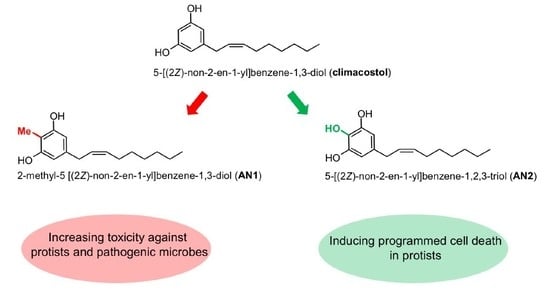
2.1. Synthesis of Climacostol and Its Analogues
2.2. Spectroscopic Analysis of Climacostol and the AN1 and AN2 Analogues
2.3. Detrimental Effects of Climacostol Analogues on Mammalian Cells
2.4. Antimicrobial Activity of Climacostol Analogues
2.5. Induction of Necrosis and Apoptosis in Ciliates
3. Discussion
3.1. Climacostol and Its Analogues
3.2. Mammalian Cells
3.3. Bacteria and Fungi
3.4. Ciliates
3.5. Cell Death in Ciliates
4. Conclusions
5. Materials and Methods
5.1. Cell Cultures
5.1.1. Mammalian Cells
5.1.2. Bacteria and Fungi
5.1.3. Ciliated Protists
5.2. Climacostol and Its Analogues
5.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
5.4. Immunofluorescence Detection of Caspase 3 Activity
5.5. Cytotoxicity Assay on Microorganisms
5.6. Necrosis/Apoptosis Detection on Ciliates
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catalani, E.; Proietti Serafini, F.; Zecchini, S.; Picchietti, S.; Fausto, A.M.; Marcantoni, E.; Buonanno, F.; Ortenzi, C.; Perrotta, C.; Cervia, D. Natural products from aquatic eukaryotic microorganisms for cancer therapy: Perspectives on anti-tumour properties of ciliate bioactive molecules. Pharmacol. Res. 2016, 113, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Buonanno, F.; Saltalamacchia, P.; Masaki, M.E.; Iio, H. Chemical defence by means of extrusive cortical granules in the heterotrich ciliate Climacostomum virens. Eur. J. Protistol. 2003, 39, 25–36. [Google Scholar] [CrossRef]
- Masaki, M.E.; Harumoto, T.; Terazima, M.N.; Miyake, A.; Usuki, Y.; Iio, H. Climacostol, a defense toxin of the heterotrich ciliate Climacostomum virens against predators. Tetrahedron Lett. 1999, 40, 8227–8229. [Google Scholar] [CrossRef]
- Fiorini, D.; Giuli, S.; Marcantoni, E.; Quassinti, L.; Bramucci, M.; Amantini, C.; Santoni, G.; Buonanno, F.; Ortenzi, C. A straightforward diastereoselective synthesis and evaluation of climacostol, a natural product with anticancer activities. Synthesis 2010, 9, 1550–1556. [Google Scholar] [CrossRef]
- Buonanno, F.; Quassinti, L.; Bramucci, M.; Amantini, C.; Lucciarini, R.; Santoni, G.; Iio, H.; Ortenzi, C. The protozoan toxin climacostol inhibits growth and induces apoptosis of human tumor cell lines. Chem. Biol. Interact. 2008, 176, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F.; Ortenzi, C. The protozoan toxin climacostol and its derivatives: Cytotoxicity studies on 10 species of free-living ciliates. Biologia 2010, 65, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Muto, Y.; Tanabe, Y.; Kawai, K.; Okano, Y.; Iio, H. Climacostol inhibits Tetrahymena motility and mitochondrial respiration. Cent. Eur. J. Biol. 2011, 6, 99–104. [Google Scholar] [CrossRef]
- Petrelli, D.; Buonanno, F.; Vitali, L.A.; Ortenzi, C. Antimicrobial activity of the protozoan toxin climacostol and its derivatives. Biologia 2012, 67, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Quassinti, L.; Ortenzi, F.; Marcantoni, E.; Ricciutelli, M.; Lupidi, G.; Ortenzi, C.; Buonanno, F.; Bramucci, M. DNA binding and oxidative DNA damage induced by climacostol–copper(II) complexes: Implications for anticancer properties. Chem. Biol. Interact. 2013, 206, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, C.; Buonanno, F.; Zecchini, S.; Giavazzi, A.; Proietti Serafini, F.; Catalani, E.; Guerra, L.; Belardinelli, M.C.; Picchietti, S.; Fausto, A.M.; et al. Climacostol reduces tumour progression in a mouse model of melanoma via the p53-dependent intrinsic apoptotic programme. Sci. Rep. 2016, 6, 27281. [Google Scholar] [CrossRef] [Green Version]
- Zecchini, S.; Proietti Serafini, F.; Catalani, E.; Giovarelli, M.; Coazzoli, M.; Di Renzo, I.; De Palma, C.; Perrotta, C.; Clementi, E.; Buonanno, F.; et al. Dysfunctional autophagy induced by the pro-apoptotic matural compound climacostol in tumour cells. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.E.; Hiro, S.; Usuki, Y.; Harumoto, T.; Terazima, M.N.; Buonanno, F.; Miyake, A.; Iio, H. Climacostol, a defense toxin of Climacostomum virens (protozoa, ciliata), and its congeners. Tetrahedron 2004, 60, 7041–7048. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bhat, S.H.; Hanif, S.; Hadi, S.M. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 2006, 580, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol–Cu(II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Wei, Q.-Y.; Cai, Y.-J.; Fang, J.-G.; Zhou, B.; Yang, L.; Liu, Z.-L. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef]
- Gembeh, S.V.; Brown, R.L.; Grimm, C.; Cleveland, T.E. Identification of chemical components of corn kernel pericarp wax associated with resistance to Aspergillus flavus infection and aflatoxin production. J. Agric. Food Chem. 2001, 49, 4635–4641. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Evidence-Based Validation of Herbal Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128008744. [Google Scholar]
- Donnelly, A.C.; Mays, J.R.; Burlison, J.A.; Nelson, J.T.; Vielhauer, G.; Holzbeierlein, J.; Blagg, B.S.J. The design, synthesis, and evaluation of Coumarin ring derivatives of the Novobiocin scaffold that exhibit antiproliferative activity. J. Org. Chem. 2008, 73, 8901–8920. [Google Scholar] [CrossRef]
- Cleary, L.; Pitzen, J.; Brailsford, J.A.; Shea, K. Progress toward the total synthesis of N-methylwelwitindolinone β isothiocyanate. Org. Lett. 2014, 16, 4480–4483. [Google Scholar] [CrossRef]
- Gomes, C.A.; Girao da Cruz, T.; Andrade, L.J.; Milhazes, N.; Borgers, F.; Marques, M.P.M. Anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Ballini, R.; Marcantoni, E.; Rosini, G. Amberlyst 15: A practical, mild and selective catalyst for methyl esterification of carboxylix acids. Synth. Commun. 1988, 18, 847–853. [Google Scholar] [CrossRef]
- Crouch, R.C.; McFayden, R.B.; Daluge, S.M.; Martin, G.E. Disentangling coupling and NOE pathways involving poorly resolved proton signals: HMQC-TOCSY and HMQC-NOESY. Magn. Reson. Chem. 1990, 28, 792–796. [Google Scholar] [CrossRef]
- Van Aller, R.M.; Clark, L.R.; Pessoney, G.F.; van Rogers, A.A.A. Prostaglandin-like fatty acid from a species in the cyperaceae. Lipids 1983, 18, 617–622. [Google Scholar] [CrossRef]
- Knodler, M.; Berardini, N.; Kanmerer, D.R.; Carle, R.; Schieber, A. Characterization of major and minor alk(en)ylresorcinols from mango (Mangifera indica L.) peels by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 945–951. [Google Scholar] [CrossRef]
- Tanaka, A.; Arai, Y.; Kim, S.-N.; Ham, J.; Usuki, T. Synthesis and biological avaluation of bilobol and adipostatin A. J. Asian Nat. Prod. Res. 2011, 13, 290–296. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Garcia-Gil, M.; Simonetti, E.; Di Giuseppe, G.; Guella, G.; Bagnoli, P.; Dini, F. Molecular mechanisms of euplotin C-induced apoptosis: Involvement of mitochondrial dysfunction, oxidative stress and proteases. Apoptosis 2007, 12, 1349–1363. [Google Scholar] [CrossRef]
- Cervia, D.; Di Giuseppe, G.; Ristori, C.; Martini, D.; Gambellini, G.; Bagnoli, P.; Dini, F. The secondary metabolite euplotin C induces apoptosis-like death in the marine ciliated protist Euplotes vannus. J. Euk. Microbiol. 2009, 56, 263–269. [Google Scholar] [CrossRef]
- Cervia, D.; Catalani, E.; Belardinelli, M.C.; Perrotta, C.; Picchietti, S.; Alimenti, C.; Casini, G.; Fausto, A.M.; Vallesi, A. The protein pheromone Er-1 of the ciliate Euplotes raikovi stimulates human T-cell activity: Involvement of interleukin-2 system. Exp. Cell. Res. 2013, 319, 56–67. [Google Scholar] [CrossRef]
- Luporini, P.; Alimenti, C.; Vallesi, A. Ciliate pheromone structures and activity: A review. Ital. J. Zool. 2015, 82, 3–14. [Google Scholar] [CrossRef]
- Pant, B.; Kato, Y.; Kumagai, T.; Matsuoka, T.; Sugiyama, M. Blepharismin produced by a protozoan Blepharisma functions as antibiotic effective against methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 1997, 155, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, R.; Harumoto, T. Defensive function of trichocysts in Paramecium tetraurelia against heterotrich ciliate Climacostomum virens. Eur. J. Protistol. 2000, 36, 415–422. [Google Scholar] [CrossRef]
- Terazima, M.N.; Harumoto, T. Defense function of pigment granules in the ciliate Blepharisma japonicum against two predatory protists, Amoeba proteus (Rhizopodea) and Climacostomum virens (Ciliata). Zool. Sci. 2004, 21, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F.; Ortenzi, C. Predator-prey interactions in ciliated protists. In Extremophilic Microbes and Metabolites—Diversity, Bioprespecting and Biotechnological Applications, 1st ed.; InTechOpen: London, UK, in press. [CrossRef]
- Nedelcu, A.M.; Driscoll, W.W.; Durand, P.M.; Herron, M.D.; Rashidi, A. On the paradigm of altruistic suicide in the unicellular world. Evolution 2011, 65, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors 2011, 4, 4–44. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero, L.; Cazzato, D.; Cervia, D.; Assi, E.; Simbari, F.; Pagni, F.; De Palma, C.; Monno, A.; Verdelli, C.; Querini, P.R.; et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 2014, 21, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, D.; Assi, E.; Moscheni, C.; Brunelli, S.; De Palma, C.; Cervia, D.; Perrotta, C.; Clementi, E. Nitric oxide drives embryonic myogenesis in chicken through the upregulation of myogenic differentiation factors. Exp. Cell Res. 2014, 320, 269–280. [Google Scholar] [CrossRef]
- Assi, E.; Cervia, D.; Bizzozero, L.; Capobianco, A.; Pambianco, S.; Morisi, F.; De Palma, C.; Moscheni, C.; Pellegrino, P.; Clementi, E.; et al. Modulation of Acid Sphingomyelinase in Melanoma Reprogrammes the Tumour Immune Microenvironment. Mediat. Inflamm. 2015. [Google Scholar] [CrossRef]
- Cervia, D.; Assi, E.; De Palma, C.; Giovarelli, M.; Bizzozero, L.; Pambianco, S.; Di Renzo, I.; Zecchini, S.; Moscheni, C.; Vantaggiato, C.; et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget 2016, 7, 24995–25009. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, C.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Campana, L.; Martelli, C.; Catalani, E.; Giovarelli, M.; Zecchini, S.; et al. Nitric Oxide Generated by Tumor-Associated Macrophages Is Responsible for Cancer Resistance to Cisplatin and Correlated With Syntaxin 4 and Acid Sphingomyelinase Inhibition. Front. Immunol. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Miyake, A.; Ishikawa, N.; Sugibayashi, R.; Zenfuku, K.; Iio, H. Chemical defense by means of pigmented extrusomes in the ciliate Blepharisma japonicum. Eur. J. Protistol. 1998, 34, 458–470. [Google Scholar] [CrossRef]
- Buonanno, F.; Anesi, A.; Guella, G.; Ortenzi, C. Blepharismins used for chemical defense in two ciliate species of the genus Blepharisma, B. stoltei and B. undulans (Ciliophora: Heterotrichida). Eur. Zool. J. 2017, 402–409. [Google Scholar] [CrossRef]
- Buonanno, F.; Anesi, A.; Di Giuseppe, G.; Guella, G.; Ortenzi, C. Chemical defense by erythrolactones in the euryhaline ciliated protist, Pseudokeronopsis erythrina. Zool. Sci. 2017, 34, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F.; Harumoto, T.; Ortenzi, C. The defensive function of trichocysts in Paramecium tetraurelia against metazoan predators compared with the chemical defense of two species of toxin-containing ciliates. Zool Sci. 2013, 30, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F.; Guella, G.; Strim, C.; Ortenzi, C. Chemical defence by mono-prenyl hydroquinone in a freshwater ciliate, Spirostomum ambiguum. Hydrobiologia 2012, 684, 97–107. [Google Scholar] [CrossRef]
- Buonanno, F. The changes in the predatory behavior of the microturbellarian Stenostomum sphagnetorum on two species of toxin-secreting ciliates of the genus Spirostomum. Biologia 2011, 66, 648–653. [Google Scholar] [CrossRef]
- Buonanno, F.; Saltalamacchia, P.; Miyake, A. Defense function of pigmentocysts in the karyorelictid ciliate Loxodes striatus. Eur. J. Protistol. 2005, 41, 151–158. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Cervia, D.; Vallesi, A. Divergences in the Response to Ultraviolet Radiation Between Polar and Non-Polar Ciliated Protozoa: UV Radiation Effects in Euplotes. Microb. Ecol. 2011, 63, 334–338. [Google Scholar] [CrossRef]
- Cervia, D.; Fehlmann, D.; Hoyer, D. Native somatostatin sst2 and sst5 receptors functionally coupled to Gi/o-protein, but not to the serum response element in AtT-20 mouse tumour corticotrophs. N.-S. Arch. Pharmacol. 2003, 367, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, D.; Repetto, A.; D’Ercole, S.; Rombini, S.; Ripa, S.; Prenna, M.; Vitali, L.A. Analysis of methicillin- susceptible and methicillin-resistant biofilm forming Staphylococcus aureus from catheter infections isolated in a large Italian hospital. J. Med. Microbiol. 2008, 57, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F. Antipredator behavior of freshwater microturbellarian Stenostomum sphagnetorum against the predatory ciliate Dileptus margaritifer. Zool. Sci. 2009, 26, 443–447. [Google Scholar] [CrossRef] [PubMed]












| Cell Line | Origin | EC50 | ||
|---|---|---|---|---|
| Climacostol | AN1 | AN2 | ||
| B16-F10 | mouse melanoma | 12.81 | 18.88 | 37.94 * |
| GL261 | mouse glioma | 28.87 | 27.32 | 21.79 |
| SK-NE-BE | human neuroblastoma | 19.13 | 34.31 * | 39.84 * |
| CT26 | mouse colon cancer | 31.58 | 36.11 | 27.42 |
| C2C12 | myoblasts of mouse | 16.19 | 29.28 * | 31.72 * |
| Compounds | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| S. aureus ATCC25923 | E. faecalis ATCC29212 | E. coli ATCC29212 | P. aeruginosa ATCC27853 | C. albicans ATCC24433 | |
| climacostol | 16 | 16 | >128 | >128 | 8 |
| AN1 | 8 | 8 | >128 | >128 | 4 |
| AN2 | 64 | 128 | >128 | >128 | 16 |
| Compounds | MBC (µg/mL) | ||||
|---|---|---|---|---|---|
| S. aureus ATCC25923 | E. faecalis ATCC29212 | E. coli ATCC29212 | P. aeruginosa ATCC27853 | C. albicans ATCC24433 | |
| climacostol | 16 | 16 | n.d. | n.d. | 8 |
| AN1 | 8 | 8 | n.d. | n.d. | 4 |
| AN2 | 128 | 128 | n.d. | n.d. | 16 |
| Ciliated Protists | Cytotoxicity (LC50 µg/mL; 95% C.I.) | |||||
|---|---|---|---|---|---|---|
| Climacostol | AN1 | AN2 | ||||
| 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | |
| B. japonicum | 3.17 | 2.04 | 2.15 | 1.55 | 4.63 | 1.94 |
| (2.79–3.94) | (1.67–2.48) | (1.82–2.54) | (1.37–1.77) | (3.92–5.47) | (1.81–2.06) | |
| E. aediculatus | 1.70 | 0.83 | 1.71 | 0.90 | 2.19 | 1.32 |
| (1.62–1.79) | (0.47–1.46) | (1.36–2.16) | (0.25–3.16) | (1.90–2.53) | (0.93–1.87) | |
| P. multimicronucleatum | 1.64 | 0.88 | 0.88 | 0.80 | 1.92 | 1.25 |
| (0.17–16.22) | (0.18–4.45) | (0.26–3.05) | (0.90–2.59) | (1.69–2.19) | (0.64–2.43) | |
| P. tetraurelia | 1.43 | 0.90 | 1.28 | 0.95 | 2.00 | 1.17 |
| (0.40–5.06) | (0.37–2.19) | (0.45–3.65) | (0.60–1.50) | (1.79–2.23) | (0.54–2.52) | |
| S. ambiguum | 2.03 | 1.66 | 1.46 | 0.64 | 3.43 | 2.04 |
| (1.81–2.27) | (1.43–1.92) | (1.16–1.85) | (0.03–15.05) | (2.80–4.19) | (1.65–2.51) | |
| S. teres | 2.04 | 1.68 | 1.28 | 0.74 | 3.50 | 1.57 |
| (1.10–3.78) | (1.55–1.83) | (0.67–2.44) | (0.03–18.21) | (3.27–3.74) | (1.39–1.86) | |
| Ciliated Protists | Effects | ||
|---|---|---|---|
| Climacostol | AN1 | AN2 | |
| B. japonicum | N | N | N |
| E. aediculatus | N | N | A |
| P. multimicronucleatum | N | N | N |
| P. tetraurelia | N | N | N |
| S. ambiguum | N | N | N |
| S. teres | N | N | N |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonanno, F.; Catalani, E.; Cervia, D.; Proietti Serafini, F.; Picchietti, S.; Fausto, A.M.; Giorgi, S.; Lupidi, G.; Rossi, F.V.; Marcantoni, E.; et al. Bioactivity and Structural Properties of Novel Synthetic Analogues of the Protozoan Toxin Climacostol. Toxins 2019, 11, 42. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010042
Buonanno F, Catalani E, Cervia D, Proietti Serafini F, Picchietti S, Fausto AM, Giorgi S, Lupidi G, Rossi FV, Marcantoni E, et al. Bioactivity and Structural Properties of Novel Synthetic Analogues of the Protozoan Toxin Climacostol. Toxins. 2019; 11(1):42. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010042
Chicago/Turabian StyleBuonanno, Federico, Elisabetta Catalani, Davide Cervia, Francesca Proietti Serafini, Simona Picchietti, Anna Maria Fausto, Simone Giorgi, Gabriele Lupidi, Federico Vittorio Rossi, Enrico Marcantoni, and et al. 2019. "Bioactivity and Structural Properties of Novel Synthetic Analogues of the Protozoan Toxin Climacostol" Toxins 11, no. 1: 42. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010042