Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Subtypes of Lingual Dystonia
2.3. Treatments
2.4. Botulinum Toxin (BoNT) Therapy
3. Discussion
3.1. Limitations of This Study
3.2. Lingual Dystonia
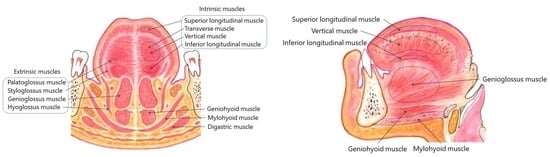
3.3. Injection Method of BoNT for Lingual Dystonia
3.4. Responses in Each Subtype
3.5. Rating Scale for Oromandibular Dystonia
3.6. Other Treatment Methods
4. Conclusions
5. Methods and Materials
5.1. Patients
5.2. Classification of Lingual Dystonia into Four Subtypes
5.3. Treatment
5.4. Botulinum Toxin (BoNT) Therapy
5.5. Individualized Injection Method for Each Subtype
5.5.1. Protrusion Type
5.5.2. Retraction Type
5.5.3. Laterotrusion Type
5.5.4. Curling Type
5.6. Evaluation of Effect
5.7. Statistical Analyses
Funding
Conflicts of Interest
References
- Berkovitz, B.K.B. Tongue. In Gray’s Anatomy, 41st ed.; Standring, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 511–517. [Google Scholar]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus up date. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.; Greene, P.E.; Fahn, S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann. Otol. Rhinol. Laryngol. 1989, 98, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Kubori, T.; Kohara, N.; Iizuka, T.; Kimura, J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia Long-term follow-up. Neurology 1999, 53, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F.; Fahn, S. Botulinum toxin injections for lingual dystonia. Laryngoscope 1991, 101, 799. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.D.; Davis, T.L.; Shannon, K.M.; Hook, M.A.; Warner, J.S. Tongue protrusion dystonia: Treatment with botulinum toxin. South Med. J. 1997, 90, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.A.; Aggarwal, A.; Bhatt, M.; Dupont, E.; Tisch, S.; Limousin, P.; Lee, P.; Quinn, N.; Bhatia, K.P. Severe tongue protrusion dystonia Clinical syndromes and possible treatment. Neurology 2006, 67, 940–943. [Google Scholar] [CrossRef]
- Kasravi, N.; Jog, M.S. Botulinum toxin in the treatment of lingual movement disorders. Mov. Disord. 2009, 24, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Esper, C.D.; Freeman, A.; Factor, S.A. Lingual protrusion dystonia: Frequency, etiology and botulinum toxin therapy. Parkinsonism Relat. Disord. 2010, 16, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, L.; Mostile, G.; Nicoletti, A.; Zappia, M.; Reggio, E.; Catania, S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J. Neurol. 2016, 263, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Clinical and phenomelogical characteristics of patients with task-specific lingual dystonia: Possible association with occupation. Front. Neurol. 2017, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.R. Galloping tongue: Post-traumatic, episodic, rhythmic movements. Neurology 1984, 34, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Ondo, W. Lingual dystonia following electrical injury. Mov. Disord. 1997, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Bader, B.; Walker, R.H.; Vogel, M.; Prosiegel, M.; McIntosh, J.; Danek, A. Tongue protrusion and feeding dystonia: A hallmark of chorea-acanthocytosis. Mov. Disord. 2010, 25, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Gollomp, S.M.; Fahn, S. Transient dystonia as a complication of varicella. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.D.; Day, L.; Dykeman, J.; Jette, N.; Pringsheim, T. The prevalence of primary dystonia: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1789–1796. [Google Scholar] [CrossRef]
- Epidemiological Study of Dystonia in Europe Collaborative Group. A prevalence study of primary dystonia in eight European countries. J. Neurol. 2000, 247, 787–792. [Google Scholar] [CrossRef]
- Duffy, P.O.; Butler, A.G.; Hawthorne, M.R.; Barnes, M.P. The epidemiology of the primary dystonias in the north of England. Adv. Neurol. 1998, 78, 121. [Google Scholar]
- Nutt, J.G.; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton, L.J., 3rd. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. [Google Scholar] [CrossRef]
- Kaji, R.; Rothwell, J.C.; Katayama, M.; Ikeda, T.; Kubori, T.; Kohara, N.; Mezaki, T.; Shibasaki, H.; Kimura, J. Tonic vibration reflex and muscle afferent block in writer’s cramp. Ann. Neurol. 1995, 38, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Kohara, N.; Katayama, M.; Kubori, T.; Mezaki, T.; Shibasaki, H.; Kimura, J. Muscle afferent block by intramuscular injection of lidocaine for the treatment of writer’s cramp. Muscle Nerve 1995, 18, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Shibasaki, H.; Iizuka, T. Factors influencing the therapeutic effect of muscle afferent block for oromandibular dystonia and dyskinesia: Implications for their distinct pathophysiology. Int. J. Oral Maxillofac. Surg. 2002, 31, 499–505. [Google Scholar] [CrossRef]
- Yoshida, K. Muskelafferentzblockierung in der Behandlung der oromandibulären Dystonie—Unterschiedliche Wirkung auf Kau- und Zungenmuskulatur-. Nervenarzt 2003, 74, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Brin, M.F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.D.; Stenner, A.; Reichel, G. Current clinical applications of botulinum toxin. Curr. Pharm. Des. 2009, 15, 3671–3680. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2017, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef]
- Ozen, B.; Gunal, D.I.; Turkmen, C.; Agan, K.; Elmaci, N.T. Speech-induced primary lingual dystonia: A rare focal dystonia. Neurol. Sci. 2011, 32, 155–157. [Google Scholar] [CrossRef]
- Hennings, J.M.H.; Krause, E.; Bötzel, K.; Wetter, T.C. Successful treatment of tardive lingual dystonia with botulinum toxin: Case report and review of the literature. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Budak, F.; Aydın, E.; Koçkaya, A.; Lbay, G. Botulinum toxin in the treatment of lingual dystonia induced by speaking. Case Rep. Neurol. 2013, 5, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Kanovsky, P.; Streitová, H.; Bares, M.; Hortová, H. Treatment of facial and orolinguomandibular dystonia by botulinum toxin A: Evidence of a long-lasting effect. Mov. Disord. 1999, 14, 886–888. [Google Scholar] [CrossRef]
- Baik, J.S.; Park, J.H.; Kim, J.Y. Primary lingual dystonia induced by speaking. Mov. Disord. 2004, 19, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Chan, L.L. Sensory tricks and treatment in primary lingual dystonia. Mov. Disord. 2005, 20, 388. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.; Singer, C. Primary focal lingual dystonia. Mov. Disord. 2006, 21, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, M.C.; Kim, J.S.; Chung, S.J.; Kim, H.J.; Kwon, M.; Shin, H.W. Lingual dystonia as a manifestation of thalamic infarction. Mov. Disord. 2009, 24, 1703–1704. [Google Scholar] [CrossRef] [PubMed]
- Felicio, A.C.; Godeiro-Junior, C.; Moriyama, T.S.; Laureano, M.R.; Felix, E.P.; Borges, V.; Silva, S.M.; Ferraz, H.B. Speech-induced lingual dystonia. Arq. Neuropsiquiatr. 2010, 68, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.; Shin, H.W.; Kim, J.S. Task specific lingual dystonia: An uncommon cause of isolated dysarthria. Commun. Sci. Disord. 2013, 18, 235–239. [Google Scholar] [CrossRef]
- Shimo, Y.; Nishida, M.; Hatano, T.; Hattori, N. Sensory tricks for isolated speech-induced lingual dystonia. BMJ Case Rep. 2015. [Google Scholar] [CrossRef]
- Choudhary, N.; Joshi, L.; Duggal, A.; Puri, V.; Khwaja, G.A. Isolated lingual involvement in Wilson’s disease. J. Neurosci. Rural. Pract. 2015, 6, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Multilingual website and cberconsultations for oromandibular dystonia. Neurol. Int. 2018, 10, 7536. [Google Scholar] [CrossRef] [PubMed]
- Slaim, L.; Cohen, M.; Klap, P.; Vidailhet, M.; Perrin, A.; Brasnu, D.; Ayache, D.; Mailly, M. Oromandibular dystonia: Demographics and clinical data from 240 patients. J. Mov. Disord. 2018, 11, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Involuntary Movements of the Stomatognathic Region. Available online: https://sites.google.com/site/oromandibulardystoniaenglish/ (accessed on 12 December 2018).
- Yoshida, K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin. Oral Investig. 2018, 22. [Google Scholar] [CrossRef]
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2017, 4, 285. [Google Scholar] [CrossRef]
- Yoshida, K. Computer-aided design/computer-assisted manufacture-derived needle guide for injection of botulinum toxin into the lateral pterygoid muscle in patients with oromandibular dystonia. J. Oral Facial Pain Headache 2018, 32, e13–e21. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hypertrophy. Toxins 2018, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031. [Google Scholar] [CrossRef]
- Yoshida, K. Surgical intervention for oromandibular dystonia-related limited mouth opening: Long-term follow-up. J. Cranioaxillofac. Surg. 2017, 45, 56–62. [Google Scholar] [CrossRef]
- Rothwell, J.C.; Obeso, J.A.; Day, B.L.; Marsden, C.D. Pathophysiology of dystonias. Adv. Neurol. 1983, 39, 851–863. [Google Scholar] [PubMed]
- Panizza, M.E.; Hallett, M.; Nilsson, J. Reciprocal inhibition in patients with hand cramps. Neurology 1989, 39, 85–89. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Thompson, P.D.; Ridding, M.C. The effect of cutaneous input on intracortical inhibition in focal task-specific dystonia. Mov. Disord. 2007, 22, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wassermann, E.M.; Canos, M.; Hallett, M. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology 1997, 49, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, A.; Hallett, M. Emerging concepts in the physiological basis of dystonia. Mov. Disord. 2013, 28, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, S.; Hanakawa, T. The curse of motor expertise: Use-dependent focal dystonia as a manifestation of maladaptive changes in body representation. Neurosci. Res. 2016, 104, 112–119. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Hamano, T.; Kohara, N.; Kimura, J.; Shibasaki, H.; Iizuka, T. Cortical potentials associated with voluntary mandibular movements. J. Dent. Res. 2000, 79, 1514–1518. [Google Scholar] [CrossRef]
- Yoshida, K.; Iizuka, T. Contingent negative variation for voluntary mandibular movements in humans. J. Oral Rehabil. 2005, 32, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Maezawa, H.; Nagamine, T.; Fukuyama, H.; Murakami, K.; Iizuka, T. Somatosensory evoked magnetic fields to air-puff on the soft palate. Neurosci. Res. 2006, 55, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, H.; Yoshida, K.; Nagamine, T.; Matsubayashi, J.; Enatsu, R.; Bessho, K.; Fukuyama, H. Somatosensory evoked magnetic fields following the tongue stimulation using needle electrodes. Neurosci. Res. 2008, 62, 131–139. [Google Scholar] [CrossRef]
- Maezawa, H.; Yoshida, K.; Matsuhashi, M.; Yokoyama, Y.; Mima, T.; Bessho, K.; Fukuyama, H. Evaluation of tongue sensory disturbance by somatosensory evoked magnetic fields following tongue stimulation. Neurosci. Res. 2011, 71, 244–250. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Kohara, N.; Murase, N.; Ikeda, A.; Shibasaki, H.; Iizuka, T. Movement-related cortical potentials jaw excursions in patients with oromandibular dystonia. Mov. Disord. 2003, 18, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Sorbo, F.D.; Comella, C.; Jinnah, H.A.; Mink, J.W.; Post, B.; Vidailhet, M.; Volkmann, J.; Warner, T.T.; Leentjens, A.F.; et al. Dystonia rating scales: Critique and recommendations. Mov. Disord. 2013, 28, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defazio, G.; Hallett, M.; Jinnah, H.A.; Stebbins, G.T.; Gigante, A.F.; Ferrazzano, G.; Conte, A.; Fabbrini, G.; Berardelli, A. Development and validation of a clinical scale for rating the severity of blepharospasm. Mov. Disord. 2015, 30, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L.; Fox, S.H.; Bhatia, K.P.; Perlmutter, J.S.; Jinnah, H.A.; Zurowski, M.; McDonald, W.M.; Marsh, L.; Rosen, A.R.; Waliczek, T.; et al. Development of the Comprehensive Cervical Dystonia Rating Scale: Methodology. Mov. Disord. Clin. Pract. 2015, 2, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, R.I.; Deakin, J.; Hawthorne, M.R. Oromandibular dystonia questionnaire (OMDQ-25): A valid and reliable instrument for measuring health-related quality of life. Clin. Otolaryngol. 2010, 35, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, G.; Magnus, R. Über die Wirkung des Novokains auf den normalen und den tetanusstarren Skelettmuskel und über die Entstehung der lokalen Muskelstarre beim Wundstarrkrampf. Pflügers Arch. Physiol. 1919, 176, 168–208. [Google Scholar] [CrossRef]
- Walshe, F. Observations on the nature of the muscular rigidity of paralysis agitans, and its relationship to tremor. Brain 1924, 47, 159–177. [Google Scholar] [CrossRef]
- Mezaki, T.; Kaji, R.; Hirota, N.; Kohara, N.; Kimura, J. Treatment of spasticity with muscle afferent block. Neurology 1999, 53, 1156–1157. [Google Scholar] [CrossRef]
- Matthews, P.; Rushworth, G. The selective effect of procaine on the stretch reflex and tendon jerk of soleus muscle when applied to its nerve. J. Physiol. 1957, 135, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Matthews, P.; Rushworth, G. The relative sensitivity of muscle nerve fibers to procaine. J. Physiol. 1957, 135, 263–269. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Takagi, A.; Iizuka, T. Customized EMG needle insertion guide for the muscle afferent block of jaw-deviation and jaw-opening dystonias. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 664–669. [Google Scholar] [CrossRef]






| Subtypes | Protrusion | Retraction | Curling | Laterotrusion | Total |
|---|---|---|---|---|---|
| Number of patients (N (%)) | 118 (68.6%) | 29 (16.9%) | 13 (7.6%) | 12 (7.0%) | 172 (100%) |
| Age (years) (mean (SD)) | 45.6 (13.8) | 45.8 (13.4) | 51.2 (15.3) | 47.67 (11.2) | 46.2 (13.7) |
| Sex (female, male) (N (%)) | 68 (57.6%) 50 (42.4%) | 17 (58.6%) 11 (41.4%) | 7 (53.8%) 6 (46.2%) | 10 (83.3%) 2 (16.7%) | 102 (59.3%) 70 (40.7%) |
| Duration of symptom (years) (mean (SD)) | 2.3 (2.6) | 2.4 (2.1) | 4.3 (4.2) | 1.8 (1.2) | 2.4 (2.6) |
| Tardive dystonia (N (%)) | 30 (25.4%) | 11 (37.8%) | 8 (61.5%) | 4 (33.3%) | 53 (30.8%) |
| Associated movement disorders (N (%)) | |||||
| Blepharospasm | 5 (2.3%) | 0 | 3 (23.1%) | 1 (8.3%) | 9 (5.2%) |
| Writer’s cramp | 3 (2.5%) | 2 (6.9%) | 1 (7.7%) | 1 (8.3%) | 7 (4.1%) |
| Cervical dystonia | 2 (2.3%) | 1 (4.0%) | 2 (15.4%) | 0 | 6 (3.5%) |
| Embouchure dystonia | 2 (1.7%) | 0 | 0 | 0 | 2 (1.2%) |
| Spasmodic dysphonia | 2 (1.7%) | 0 | 0 | 0 | 2 (1.2%) |
| Subtype of oromandibular dystonia (N (%)) | |||||
| Jaw-opening dystonia | 13 (11.0%) | 4 (13.8.0%) | 0 | 2 (16.7%) | 19 (11.0%) |
| Jaw-closing dystonia | 4 (3.4%) | 3 (10.3%) | 3 (23.1%) | 1 (8.3%) | 10 (5.8%) |
| Jaw-deviation dystonia | 2 (1.7%) | 0 | 0 | 2 (16.7%) | 4 (2.3%) |
| Jaw-protrusion dystonia | 1 (0.8%) | 0 | 0 | 0 | 1 (0.6%) |
| Lip dystonia | 1 (0.8%) | 0 | 0 | 0 | 1 (0.6%) |
| Stereotypy (N (%)) | 118 (100%) | 29 (100%) | 13 (100%) | 12 (100%) | 172 (100%) |
| Task-specificity (N (%)) | 113 (95.8%) | 28 (96.6%) | 6 (46.2%) | 8 (66.7%) | 155 (90.1%) |
| Sensory tricks (N (%)) | 85 (72.0%) | 17 (58.6%) | 9 (69.2%) | 6 (50.0%) | 117 (68.2%) |
| Morning benefit (N (%)) | 92 (78.0%) | 22 (75.9%) | 4 (30.8%) | 4 (33.3%) | 122 (70.9%) |
| Subtypes | Protrusion | Retraction | Curling | Laterotrusion | Total | |
|---|---|---|---|---|---|---|
| Number of patients | 106 | 12 | 10 | 8 | 136 | |
| Age (years) (mean (SD)) | 45.6 (13.6) | 44.5 (11.2) | 54.4 (16.2) | 51.8 (7.9) | 46.5 (13.5) | |
| Sex (female, male) (N (%)) | 58 (54.7%) 48 (45.3%) | 6 (50.0%) 6 (50.0%) | 6 (60.0%) 4 (40.0%) | 6 (75.0%) 2 (25.0%) | 74 (54.4%) 62 (45.6%), | |
| BoNT injection (times) (mean (SD)) | 4.9 (3.5) | 2.5 (2.0) | 5.6 (7.1) | 6.3 (4.7) | 4.8 (3.9) | |
| Comprehensive improvement (%) (mean (SD)) | 78.7 (14.2) | 67.9 (10.2) | 81.9 (35.5) | 73.2 (12.6) | 77.6 (16.7) | |
| Subjective improvement (%) (mean (SD)) | 79.3 (13.5) | 74.2 (9.0) | 85.0 (36.8) | 76.3 (11.6) | 79.0 (16.0) | |
| Adverse effects | per patient (%) (mean (SD)) | 14 (13.2%) | 2 (16.7%) | 0 | 1 (12.5%) | 17 (12.5%) |
| per session (%) (mean (SD)) | 21 (4.1%) | 2 (6.7%) | 0 | 1 (2.0%) | 24 (3.7%) | |
| Scores | Baseline | After BoNT Therapy | p-Value |
|---|---|---|---|
| Mastication (points) (mean (SD)) | 0.81 (0.97) | 0.25 (0.53) | p < 0.001 |
| Speech (points) (mean (SD)) | 2.8 (0.81) | 0.73 (0.51) | p < 0.001 |
| Pain (points) (mean (SD)) | 0.69 (1.0) | 0.1 (0.32) | p < 0.001 |
| Discomfort (points) (mean (SD)) | 2.51 (0.72) | 0.49 (0.54) | p < 0.001 |
| Total (points) (mean (SD)) | 6.9 (2.4) | 1.6 (1.3) | p < 0.001 |
| Subtypes | Protrusion | Retraction | Curling | Laterotrusion | |
|---|---|---|---|---|---|
| Doses [units] | 15–60 | 15–50 | 10–40 | 10–40 | |
| Main muscles | Bilateral genioglossus muscles (50–100% of total dose) | Bilateral genioglossus muscles (30–70% of total dose) | Bilateral superior longitudinal muscles (100% of total dose) | Superior and inferior longitudinal muscles on the deviated side (70–100% of total dose) | |
| Additional muscles | With laterotrusion | Superior and inferior longitudinal muscles on the deviated side | Contracted muscles based on electromyography (EMG) examination including intrinsic and geniohyoid muscles | - | Genioglossus muscle on the opposite side of deviation |
| With curling | Bilateral superior longitudinal muscles | ||||
| With flattening | Bilateral vertical muscles | ||||
| With elongation | Bilateral transverse muscles | ||||
| Points | Mastication Scale | Speech Scale | Pain Scale | Discomfort Scale |
|---|---|---|---|---|
| 4 | Only able to consume liquids | Inaudible (more than 50% of speech) | Severe pain (visual analog scale score: >75%) | Severe discomfort |
| 3 | Finds it difficult and takes a long time to eat soft food | Inaudible (less than 50% of speech) | Moderate, intermittent to continuous pain (visual analog scale score: 50–75%) | Moderate to severe discomfort |
| 2 | Only able to eat soft food | Audible, but difficult to comprehend | Mild continuous to moderate intermittent pain (visual analog scale score: 25–50%) | Mild to moderate discomfort |
| 1 | Able to eat anything, but it takes a long time | Finds it hard to speak clearly | Mild, intermittent pain (visual analog scale score: <25%) | Mild discomfort |
| 0 | Normal | Normal | No pain | No discomfort |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K. Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features. Toxins 2019, 11, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010051
Yoshida K. Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features. Toxins. 2019; 11(1):51. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010051
Chicago/Turabian StyleYoshida, Kazuya. 2019. "Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features" Toxins 11, no. 1: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010051