Endophyte Infection and Methyl Jasmonate Treatment Increased the Resistance of Achnatherum sibiricum to Insect Herbivores Independently
Abstract
:1. Introduction
2. Results
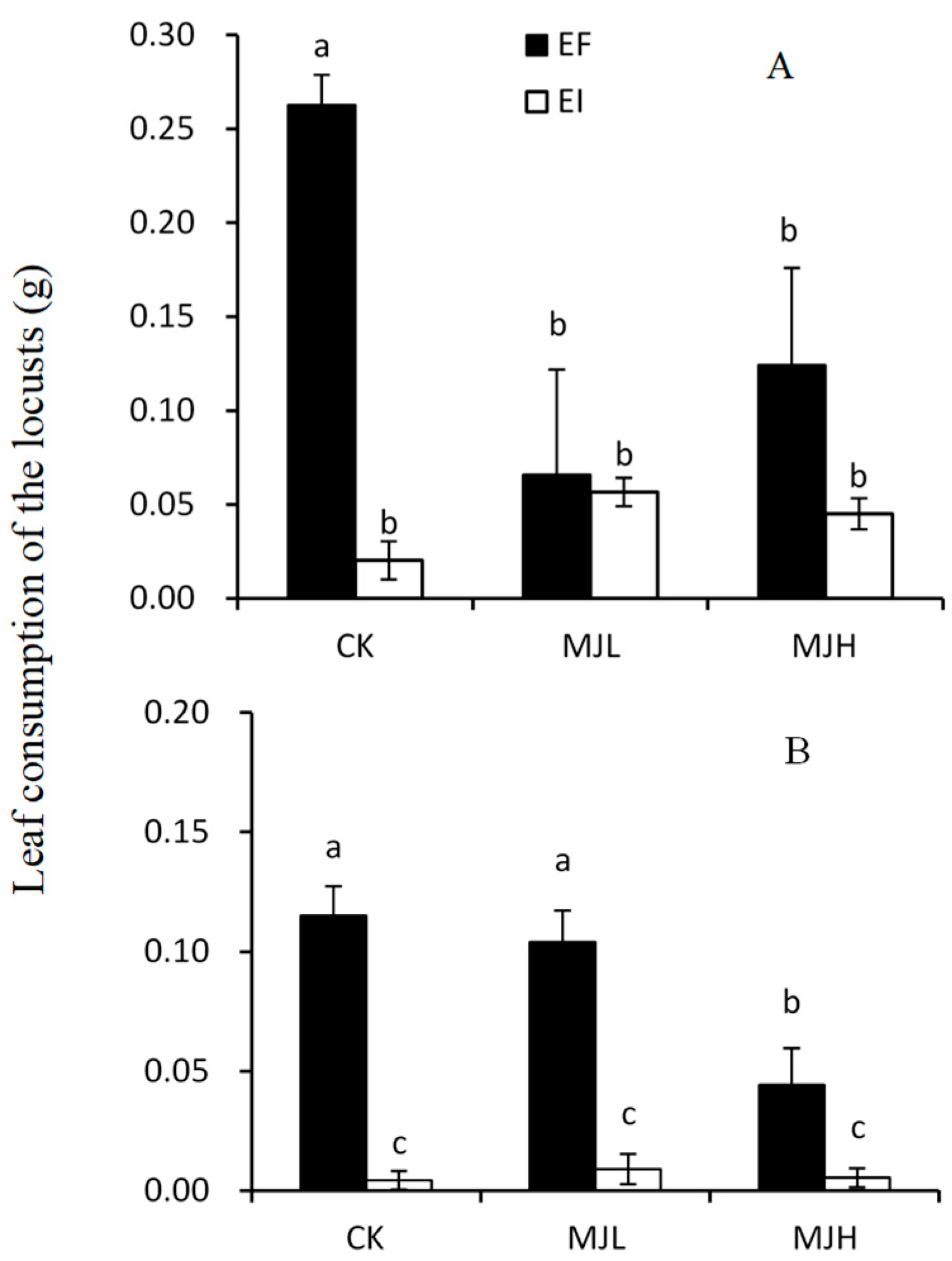
2.1. Leaf Consumption by L. migratoria
2.2. Growth and Biomass
2.3. Physiological Variables
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Treatment
5.2. Locusta Migratoria
5.3. Methyl Jasmonate (MJ) Treatment
5.4. Choice Feeding Experiment
5.5. No-Choice Feeding Experiment
5.6. Growth and Biomass
5.7. Physiological Variables
5.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, G. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1992, 1, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Wali, P.; Helander, M.; Faeth, S.H. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Clay, K. Fungal endophytes of grasses. Annu. Rev. Ecol. Syst. 1990, 21, 275–297. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Endophyte infection enhances the ability of tall fescue to untilize sparingly available phosphorus. J. Plant Nutr. 1999, 22, 835–853. [Google Scholar] [CrossRef]
- Hesse, U.; Schöberlein, W.; Wittenmayer, L.; Förster, K.; Warnstorff, K.; Diepenbrock, W.; Merbach, W. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Sci. 2003, 58, 407–415. [Google Scholar] [CrossRef]
- Burns, J.C.; Fisher, D.S. Intake and digestion of ‘Jesup’ tall fescue hays with a novel fungal endophyte, without an endophyte, or with a wild-type endophyte. Crop. Sci. 2006, 46, 216–223. [Google Scholar] [CrossRef]
- Gibert, A.; Hazard, L. Endophyte infection of Festuca eskia enhances seedling survival to drought and cutting at the expense of clonal expansion. J. Plant Ecol. 2011, 4, 201–208. [Google Scholar] [CrossRef]
- Worchel, E.R.; Giauque, H.E.; Kivlin, S.N. Fungal symbionts alter plant drought response. Microb. Ecol. 2013, 65, 671–678. [Google Scholar] [CrossRef]
- Rúa, M.A.; Mcculley, R.L.; Mitchell, C.E. Fungal endophyte infection and host genetic background jointly modulate host response to an aphid-transmitted viral pathogen. J. Ecol. 2013, 101, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fannin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3315. [Google Scholar] [CrossRef] [PubMed]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D. Lolitrems, peramine and paxilline: Mycotoxins of the ryegrass/endophyte interaction. Agric. Ecosyst. Environ. 1993, 44, 103–122. [Google Scholar] [CrossRef]
- Siegel, M.R.; Bush, L.P. Toxin production in grass/endophyte associations. In Plant Relationships. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Carroll, G.C., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 185–207. [Google Scholar]
- Siegel, M.R.; Bush, L.P. Defensive chemicals in grass-fungal endophyte associations. In Phytochemical Diversity and Redundancy in Ecological Interactions. Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Springer: Boston, MA, USA, 1996; pp. 81–119. [Google Scholar]
- Rowan, D.D.; Latch, G.C.M. Utilization of endophyte-infected perennial ryegrass for increased insect resistance. In Biotechnology of Endophytic Fungi of Grasses; CRC Press: Boca Raton, FL, USA, 1994; pp. 169–183. [Google Scholar]
- Faeth, S.H.; Shochat, E. Inherited microbial symbionts increase herbivore abundances and alter arthropod diversity on a native grass. Ecology 2010, 91, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Blande, J.D.; Gundel, P.E.; Helander, M.; Saikkonen, K. Epichloë endophytes alter inducible indirect defences in host grasses. PLoS ONE 2014, 9, e101331. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Loon, L.C.V.; Pieterse, C.M.J. Jasmonates-signals in plant-microbe interactions. J. Plant Growth Regul. 2004, 23, 211–222. [Google Scholar]
- Kiers, T.E.; Adler, L.S.; Grman, E.L.; Van Der Heijden, M.G.A. Manipulating the jasmonate response: How do methyl jasmonate additions mediate characteristics of aboveground and belowground mutualisms? Funct. Ecol. 2010, 24, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.A.R.; Arce, C.C.M.; Ferrieri, A.P.; Baldwin, I.T.; Matthias, E. Jasmonate-dependent depletion of soluble sugars compromises plant resistance to Manduca sexta. New Phytol. 2015, 207, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Balbi, V.; Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2008, 177, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Baldwin, I.T. Jasmonates and related compounds in plant-insect interactions. J. Plant Growth Regul. 2004, 23, 238–245. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Jasmonate-induced defenses: A tale of intelligence, collaborators and rascals. Trends Plant. Sci. 2011, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Xue, X.; Ren, S.X.; Cuthbertson, A.G.S.; Dam, N.M.V.; Qiu, B.L. Root and shoot jasmonic acid induced plants differently affect the performance of Bemisia tabaci and its parasitoid Encarsia formosa. Basic Appl. Ecol. 2013, 14, 670–679. [Google Scholar] [CrossRef]
- Mcconn, M.; Creelman, R.A.; Bell, E.; Mullet, J.E.; Browse, J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 5473–5477. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, C.; Lee, G.I.; Howe, G.A. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl. Acad. Sci. USA 2002, 99, 6416. [Google Scholar] [CrossRef]
- Qiu, B.L.; Jeffreya, H.; Ciskae, R.; Louiseem, V.; Nicolem, V.D. Nonlinear effects of plant root and shoot jasmonic acid application on the performance of Pieris brassicae and its parasitoid Cotesia glomerata. Funct. Ecol. 2009, 23, 496–505. [Google Scholar] [CrossRef]
- Simons, L.; Bultman, T.L.; Sullivan, T.J. Effects of methyl Jasmonate and an endophytic fungus on plant resistance to insect herbivores. J. Chem. Ecol. 2008, 34, 1511–1517. [Google Scholar] [CrossRef]
- Moore, J.P.; Paul, N.D.; Whittaker, J.B.; Taylor, J.E. Exogenous jasmonic acid mimics herbivore-induced systemic increase in cell wall bound peroxidase activity and reduction in leaf expansion. Funct. Ecol. 2003, 17, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jones, A.D.; Howe, G.A. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschold, A.; Halitschke, R.; Baldwin, I.T. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J. 2007, 51, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hedhili, S.; Montiel, G.; Zhang, Y.; Chatel, G.; Pré, M.; Gantet, P.; Memelink, J. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant. J. 2011, 67, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, A.Z.; Wei, Y.K.; Lin, F.; Li, C.; Liu, Z.J.; Gao, Y.B. Taxonomy, diversity and origins of symbiotic endophytes of Achnatherum sibiricum in the Inner Mongolia Steppe of China. FEMS Microbiol. Lett. 2009, 301, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Zhu, M.; Qin, J.; Ren, A.; Gao, Y. Stroma-bearing endophyte and its potential horizontal transmission ability in Achnatherum sibiricum. Mycologia 2015, 107, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.J.; Rodstrom, J.; Vandop, J.; Librizzi, J.; Graham, C.; Schardl, C.L.; Bultman, T.L. Symbiont-mediated changes in Lolium arundinaceum inducible defenses: Evidence from changes in gene expression and leaf composition. New Phytol. 2007, 176, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Faulkner, J.R.; Florea, S.; Pan, J. Chemotypic diversity of epichloae, fungal symbionts of grasses. Fungal Ecol. 2012, 5, 331–344. [Google Scholar] [CrossRef]
- Saikkonen, K.; Gundel, P.E.; Helander, M. Chemical ecology mediated by fungal endophytes in grasses. J. Chem. Ecol. 2013, 39, 962–968. [Google Scholar] [CrossRef]
- Wilkinson, H.H.; Siegel, M.R.; Blankenship, J.D.; Mallory, A.C.; Bush, L.P.; Schardl, C.L. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Mol. Plant Microbe Interact. 2000, 13, 1027–1033. [Google Scholar] [CrossRef]
- Prestidge, R.A.; Gallagher, R.T. Endophyte fungus confers resistance to ryegrass: Argentine stem weevil larval studies. Ecol. Entomol. 1988, 13, 429–435. [Google Scholar] [CrossRef]
- Shymanovich, T.; Saari, S.; Lovin, M.E.; Jarmusch, A.K.; Jarmusch, S.A.; Musso, A.M.; Charlton, N.D.; Young, C.A.; Cech, N.B.; Faeth, S.H. Alkaloid variation among epichloid endophytes of sleepygrass (Achnatherum robustum) and consequences for resistance to insect herbivores. J. Chem. Ecol. 2015, 41, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Ballare, C.L.; Scopel, A.L.; Stapleton, A.E.; Yanovsky, M.J. Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant. Physiol. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Izaguirre, M.M.; Mazza, C.A.; Svatos, A.; Baldwin, I.T.; Ballaré, C.L. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuate and Nicotiana longiflora. Ann. Bot. 2007, 99, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Parsons, A.J.; Fraser, K.; Xue, H.; Newman, J.A. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol. 2008, 146, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Pańka, D.; Piesik, D.; Jeske, M.; Baturo-Cieśniewska, A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J. Plant Physiol. 2013, 170, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Alloush, G.A.; Belesky, D.P. Evidence for chemical changes on the root surface of tall fescue in response to infection with the fungal endophyte Neotyphodium coenophialum. Plant Soil 1998, 205, 1–12. [Google Scholar] [CrossRef]
- Karban, R.; Kuc, J. Induced resistance against pathogens and herbivores: An overview. In Induced Plant Defenses Against Pathogens and Herbivores; Agrawal, A.A., Ed.; APS Press: St. Paul, MN, USA, 1999; pp. 1–15. [Google Scholar]
- Mercedes, E.C.; Sylvia, V.C.; Victor, H.A. Relationships between aalicylic acid content, phenylalanine ammonia-lyase (PAL) activity, and resistance of barley to aphid infestation. J. Agric. Food Chem. 2003, 51, 2227–2231. [Google Scholar]
- Tang, Y.; Zou, J.; Zhang, L.; Li, Z.; Ma, C.; Ma, N. Anti-fungi activities of Bacillus thuringiensis H3 chitinase and immobilized chitinase particles and their effects to rice seedling defensive enzymes. J. Nanosci. Nanotechnol. 2012, 12, 8081–8086. [Google Scholar] [CrossRef]
- Haruta, M.; Pedersen, J.A.; Constabel, C.P. Polyphenol oxidase and herbivore defense in trembling aspen (Populus tremuloides): CDNA cloning, expression, and potential substrates. Physiol. Plant 2001, 112, 552–558. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Fan, Z.; Hua, B.Z. Enhancement of phenylalanine ammonia lyase, polyphenoloxidase, and peroxidase in cucumber seedlings by Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) infestation. J. Integr. Agric. 2008, 7, 82–87. [Google Scholar] [CrossRef]
- Constabel, C.P.; Yip, L.; Patton, J.J.; Christopher, M.E. Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiol. 2000, 124, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Turner, J.G. Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 2008, 3, e3699. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, M.; Posthumus, M.A.; Mumm, R.; Mueller, M.J.; Loon, J.J.A.V.; Dicke, M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: Effects of time and dose, and comparison with induction by herbivores. J. Exp. Bot. 2009, 60, 2575–2587. [Google Scholar] [CrossRef]
- Matsuura, H.; Aoi, A.; Satou, C.; Nakaya, M.; Masuta, C.; Nabeta, K. Simultaneous UPLC MS/MS analysis of endogenous jasmonic acid, salicylic acid, and their related compounds. Plant. Growth Regul. 2009, 57, 293–301. [Google Scholar] [CrossRef]
- Henkes, G.J.; Thorpe, M.R.; Minchin, P.E.; Schurr, U.; Roese, U.S. Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an hour. Plant Cell Environ. 2008, 31, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.P.; Baldwin, I.T. Transport of [2- 14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 1997, 203, 436–441. [Google Scholar]
- Staswick, P.E.; Su, W.; Howell, S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ayoubi, P.; Weng, H.; Palmer, D.A.; Mitchell, R.E.; Jones, W.; Bender, C.L. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005, 42, 201–217. [Google Scholar] [CrossRef] [Green Version]
- van Kleunen, M.; Ramponi, G.; Schmid, B. Effects of herbivory simulated by clipping and jasmonic acid on Solidago canadensis. Basic Appl. Ecol. 2004, 5, 173–181. [Google Scholar] [CrossRef]
- Cho, K.; Agrawal, G.K.; Shibato, J.; Jung, Y.H.; Kim, Y.K.; Nahm, B.H.; Jwa, N.S.; Tamogami, S.; Han, O.; Kohda, K.; et al. Survey of differentially expressed proteins and genes in jasmonic acid treated rice seedling shoot and root at the proteomics and transcriptomics levels. J. Proteome Res. 2007, 6, 3581–3603. [Google Scholar] [CrossRef]
- Walls, R.; Appel, H.; Cipollini, M.; Schultz, J. Fertility, root reserves and the cost of inducible defenses in the perennial plant Solanum carolinense. J. Chem. Ecol. 2005, 31, 2263–2288. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, E.; Miyamoto, K.; Saniewski, M.; Ueda, J. Jasmonates are essential factors inducing gummosis in tulips: Mode of action of jasmonates focusing on sugar metabolism. J. Plant Physiol. 2005, 162, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Babst, B.A.; Ferrieri, R.A.; Gray, D.W.; Lerdau, M.; Schlyer, D.J.; Schueller, M.; Thorpe, M.R.; Orians, C.M. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol. 2005, 167, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanik, N.; Gomez, S.; Best, M.; Schueller, M.; Orians, C.M.; Ferrieri, R.A. Partitioning of new carbon as C-11 in Nicotiana tabacum reveals insight into methyl jasmonate induced changes in metabolism. J. Chem. Ecol. 2010, 36, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, T.O.; Kjf, V.; Jansen, J.J.; Raaijmakers, C.E.; Bakxschotman, T.; Mcintyre, L.M.; Wh, V.D.P.; Biere, A.; van Dam, N.M. Correction: Plants know where it hurts: Root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS ONE 2013, 8, e65502. [Google Scholar] [CrossRef]
- van Dam, N.M.; Oomen, W.A.T. Root and shoot jasmonic acid applications differentially affect leaf chemistry and herbivore growth. Plant Signal. Behav. 2008, 3, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Quaghebeur, H.; Felton, G.W. Reiterative and interruptive signaling in induced plant resistance to chewing insects. Phytochemistry 2011, 72, 1624–1634. [Google Scholar] [CrossRef]
- Pineda, A.; Dicke, M.; Pieterse, C.M.J.; Pozo, M.J. Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Funct. Ecol. 2013, 27, 574–586. [Google Scholar] [CrossRef]
- de Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Van Loon, L.C.; Dicke, M. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [PubMed]
- Herrera-Medina, M.J.; Tamayo, M.I.; Vierheilig, H.; Ocampo, J.A.; García-Garrido, J.M. The jasmonic acid signalling pathway restricts the development of the arbuscular mycorrhizal association in tomato. J. Plant Growth Regul. 2008, 27, 221–230. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R.S. Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef]
- Jacobs, S.; Zechmann, B.; Molitor, A.; Trujillo, M.; Petutschnig, E.; Likpa, V.; Kogel, K.H.; Schäfer, P. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis Roots by the fungus Piriformospora indica. Plant Physiol. 2011, 156, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Barazani, O.; Benderoth, M.K.; Kuhlemeier, C.; Baldwin, I.T. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 2005, 146, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Cosme, M.; Lu, J.; Erb, M.; Stout, M.J.; Franken, P.; Wurst, S. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 2016, 211, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, J.K.; Bacon, C.W.; Cutler, H.G.; Arrendale, R.F.; Robbins, J.D. In vitro auxin production by Balansia epichloë. Phytochemistry 1985, 24, 1429–1431. [Google Scholar] [CrossRef]
- De Battista, J.P.D.; Bacon, C.W.; Severson, R.; Plattner, R.D.; Bouton, J.H. Indole acetic acid production by the fungal endophyte of tall fescue. Agron. J. 1990, 82, 878–880. [Google Scholar] [CrossRef]
- Yue, Q.; Miller, C.J.; White, J.F.; Richardson, M.D. Isolation and characterization of fungal inhibitors from Epichloë festucae. J. Agric. Food Chem. 2000, 48, 4687–4692. [Google Scholar] [CrossRef]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 1192–1200. [Google Scholar] [CrossRef]
- Matschi, S.; Hake, K.; Herde, M.; Hause, B.; Romeis, T. The calcium-dependent protein kinase CPK28 regulates development by inducing growth phase-specific, spatially restricted alterations in jasmonic acid levels independent of defense responses in Arabidopsis. Plant Cell 2015, 27, 591–606. [Google Scholar] [CrossRef]
- Heinrich, M.; Hettenhausen, C.; Lange, T.; Wünsche, H.; Fang, J.; Baldwin, I.T.; Wu, J. High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuata stems. Plant J. 2013, 73, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.G.; Dai, C.C. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Latch, G.C.M.; Christensen, M.J.; Samuels, G.J. Five endophytes of Lolium and Festuca in New Zealand. Mycotaxon 1984, 20, 535–550. [Google Scholar]
- Kannadan, S.; Rudgers, J.A. Endophyte symbiosis benefits a rare grass under low water availability. Funct. Ecol. 2008, 22, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stat. 1938, 347, 35–37. [Google Scholar]
- Zhou, Y.; Li, X.; Gao, Y.; Liu, H.; Gao, Y.B.; van der Heijden, M.G.A.; Ren, A.Z. Plant endophytes and arbuscular mycorrhizal fungi alter plant competition. Funct. Ecol. 2018, 32, 1168–1179. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Yu, L.M.; Wang, C.K.; Wang, X.C. Allocation of nonstructural carbohydrates for three temperate tree species in Northeast China. Chin. J. Plant Ecol. 2011, 35, 1245–1255. [Google Scholar] [CrossRef]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.A.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef] [Green Version]



| Treatment | Plant Height | Leaf Number | Tiller Number | Shoot Biomass | Root Biomass | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Endophyte (E) | 2.155 | 0.155 | 0.070 | 0.793 | 22.617 | <0.01 | 11.170 | <0.01 | 17.078 | <0.01 |
| MJ | 12.007 | <0.01 | 35.715 | <0.01 | 181.574 | <0.01 | 24.986 | <0.01 | 0.350 | 0.708 |
| E × MJ | 1.199 | 0.319 | 3.449 | 0.048 | 19.904 | <0.01 | 3.832 | 0.036 | 3.145 | 0.061 |
| Treatment | Soluble Sugar | Total Phenolics | Phenylalanine Ammonia Lyase (PAL) | Polyphenol Oxidase (PPO) | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| No feeding | ||||||||
| Endophyte (E) | 6.631 | 0.017 | 5.656 | 0.026 | 7.841 | 0.012 | 3.797 | 0.063 |
| MJ | 7.212 | 0.004 | 2.908 | 0.075 | 10.215 | 0.001 | 5.012 | 0.015 |
| E × MJ | 0.111 | 0.896 | 0.964 | 0.396 | 2.790 | 0.088 | 0.671 | 0.520 |
| Feeding | ||||||||
| E | 8.529 | 0.008 | 2.555 | 0.124 | 10.780 | 0.003 | 6.589 | 0.019 |
| MJ | 0.572 | 0.573 | 0.215 | 0.808 | 1.377 | 0.272 | 1.735 | 0.205 |
| E × MJ | 0.651 | 0.531 | 0.466 | 0.634 | 2.043 | 0.152 | 0.347 | 0.711 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Wu, M.; Liu, H.; Gao, Y.; Ren, A. Endophyte Infection and Methyl Jasmonate Treatment Increased the Resistance of Achnatherum sibiricum to Insect Herbivores Independently. Toxins 2019, 11, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010007
Qin J, Wu M, Liu H, Gao Y, Ren A. Endophyte Infection and Methyl Jasmonate Treatment Increased the Resistance of Achnatherum sibiricum to Insect Herbivores Independently. Toxins. 2019; 11(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010007
Chicago/Turabian StyleQin, Junhua, Man Wu, Hui Liu, Yubao Gao, and Anzhi Ren. 2019. "Endophyte Infection and Methyl Jasmonate Treatment Increased the Resistance of Achnatherum sibiricum to Insect Herbivores Independently" Toxins 11, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11010007




