Cytotoxicity of Deoxynivalenol after Being Exposed to Gaseous Ozone
Abstract
:1. Introduction
2. Results
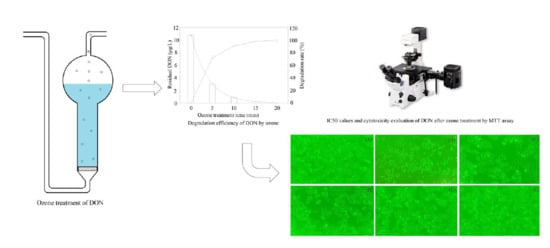
2.1. Change of DON during Ozone Exposure
2.2. Cytotoxicity of DON after Ozone Exposure
3. Discussion
4. Materials and Methods
4.1. Materials and Cells
4.2. Determination of DON by HPLC-UV
4.3. Ozone Treatment of DON in Aqueous Solution
4.4. Cell Culture and MTT Assay
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, J.; Wang, Q.C.; Zhu, C.C.; Liu, J.; Zhang, Y.; Cui, X.S.; Kim, N.H.; Sun, S.C. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol. 2016, 300, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes dos Santos, J.; Souza, T.M.; Ono, E.Y.S.; Hashimoto, E.H.; Bassoi, M.C.; de Miranda, M.Z.; Itano, E.N.; Kawamura, O.; Hirooka, E.Y. Natural occurrence of deoxynivalenol in wheat from Paraná State, Brazil and estimated daily intake by wheat products. Food Chem. 2013, 138, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kuča, K.; Humpf, H.U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2016, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tripathi, A.; Chaudhari, B.P.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Deoxynivalenol induced mouse skin cell proliferation and inflammation via MAPK pathway. Toxicol. Appl. Pharmacol. 2014, 279, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.U. Thermal degradation of the Fusarium mycotoxin deoxynivalenol. J. Agric. Food. Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef]
- Ikunaga, Y.; Sato, I.; Grond, S.; Numaziri, N.; Yoshida, S.; Yamaya, H.; Hiradate, S.; Hasegawa, M.; Toshima, H.; Koitabashi, M.; et al. Nocardioides sp. strain WSNO5-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl. Microbiol. Biotechnol. 2011, 89, 419–427. [Google Scholar] [CrossRef]
- Sun, C.; Ji, J.; Wu, S.L.; Sun, C.P.; Pi, F.W.; Zhang, Y.Z.; Tang, L.L.; Sun, X.L. Saturated aqueous ozone degradation of deoxynivalenol and its application in contaminated grains. Food Control 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Bittencourt, K.O.; Scussel, V.M. Ozone treatment efficiency on Fusarium graminearum and deoxynivalenol degradation and its effects on whole wheat grains (Triticum aestivum L.) quality and germination. J. Stored Prod. Res. 2014, 59, 245–253. [Google Scholar] [CrossRef]
- Wang, L.; Shao, H.L.; Luo, X.H.; Wang, R.; Li, Y.F.; Li, Y.N.; Luo, Y.P.; Chen, Z.X. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Mckenzie, K.S.; Sarr, A.B.; Mayura, A.K.; Bailey, R.H.; Miller, D.R.; Rogers, T.D.; Norred, W.P.; Voss, K.A.; Plattner, R.D.; Kubena, L.F.; et al. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem. Toxicol. 1997, 35, 807–820. [Google Scholar] [CrossRef]
- Piemontese, L.; Messia, M.C.; Marconi, E.; Falasca, L.; Zivoli, R.; Gambacorta, L.; Perrone, G.; Solfrizzo, M. Effect of gaseous ozone treatments on DON, microbial contaminants and technological parameters of wheat and semolina. Food Addit. Contam. 2018, 35, 761–772. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Shao, H.L.; Luo, X.H.; Wang, R.; Li, Y.F.; Li, Y.N.; Luo, Y.P.; Zhang, D.J.; Chen, Z.X. In vivo toxicity assessment of deoxynivalenol-contaminated wheat after ozone degradation. Food Addit. Contam. 2016, 34, 103–112. [Google Scholar] [CrossRef]
- Diao, E.J.; Hou, H.H.; Dong, H.Z. Ozonolysis mechanism and influencing factors of aflatoxin B1: A review. Trends Food Sci. Tech. 2013, 33, 21–26. [Google Scholar] [CrossRef]
- Christ, D.; Savi, G.D.; Scussel, V.M. Effectiveness of ozone gas application methods against combined multi-contaminants in food. Food Public Health 2017, 7, 51–58. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of ozone treatment on deoxynivalenol and quality evaluation of ozonized wheat. Food Addit. Contam. 2015, 32, 544–553. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Bensassi, F.; El Golli-Bennour, E.; Abid-Essefi, S.; Bouaziz, C.; Hajlaoui, M.R.; Bacha, H. Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 2009, 264, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Cetin, Y.; Bullerman, L.B. Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem. Toxicol. 2005, 43, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Königs, M.; Lenczyk, M.; Schwerdt, G.; Holzinger, H.; Gekle, M.; Humpf, H.U. Cytotoxicity, metabolism and cellular uptake of the mycotoxin deoxynivalenol in human proximal tubule cells and lung fibroblasts in primary culture. Toxicology 2007, 240, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Casteel, M.; Didier, A.; Dietrich, R.; Märtlbauer, E. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 2009, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Franzova, P.; Juan-García, A.; Font, G. Toxicological interactions between the mycotoxins beauvericin, deoxynivalenol and T-2 toxin in CHO-K1 cells in vitro. Toxicon 2011, 58, 315–326. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.J. Cytotoxic effects induced by patulin, deoxynivalenol and toxin T2 individually and in combination in hepatic cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef]
- Savard, C.; Provost, C.; Alvarez, F.; Pinilla, V.; Music, N.; Jacques, M.; Gagnon, C.A.; Chorfi, Y. Effect of deoxynivalenol (DON) mycotoxin on in vivo and in vitro porcine circovirus type 2 infections. Vet. Microbiol. 2015, 176, 257–267. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, D.; Diao, E.; Hou, H.; Xie, P.; Mao, R.; Dong, H.; Qian, S. Cytotoxicity of Deoxynivalenol after Being Exposed to Gaseous Ozone. Toxins 2019, 11, 639. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110639
Ren D, Diao E, Hou H, Xie P, Mao R, Dong H, Qian S. Cytotoxicity of Deoxynivalenol after Being Exposed to Gaseous Ozone. Toxins. 2019; 11(11):639. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110639
Chicago/Turabian StyleRen, Dongliang, Enjie Diao, Hanxue Hou, Peng Xie, Ruifeng Mao, Haizhou Dong, and Shiquan Qian. 2019. "Cytotoxicity of Deoxynivalenol after Being Exposed to Gaseous Ozone" Toxins 11, no. 11: 639. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110639