Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Samples and Homogeneity Testing
2.2. Participants Instrumental Method Setup
2.3. Laboratory Qualification
2.4. Full Collaborative Study
2.5. Output: Future Perspective
3. Conclusions
4. Materials and Methods
4.1. Study Organization
4.1.1. Study Materials
4.1.2. Study Samples
4.1.3. Study Analytical Standards Solutions for Spiking Purposes
4.1.4. Study Analytical Standard Solutions for External Calibration Curves
4.1.5. Shipping Study Materials
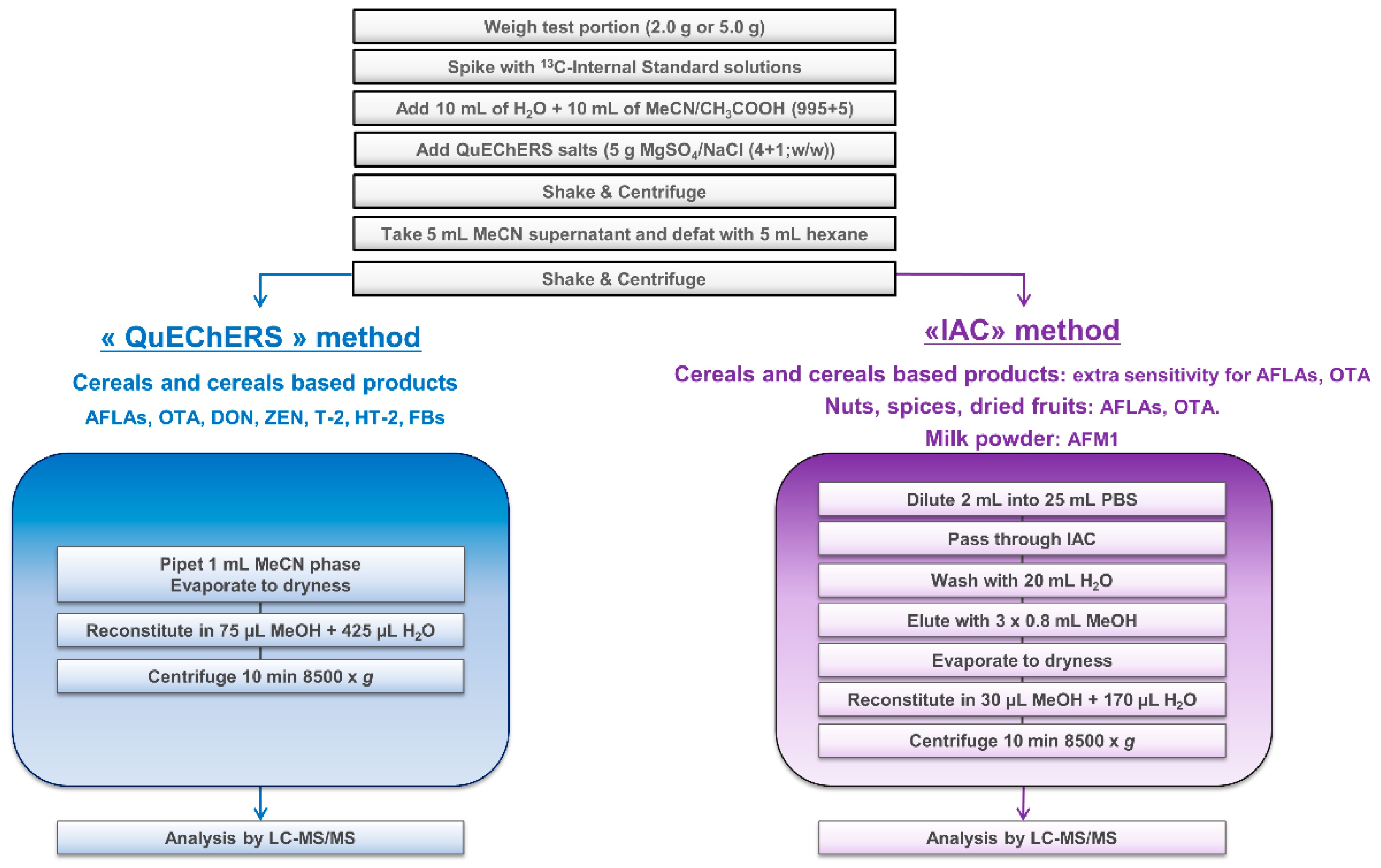
4.2. Sample Preparation
- “QuEChERS”: Generic cleanup for all mycotoxins potentially present in cereals when an improved sensitivity for AFLAs and OTA is not required. An aliquot of the defatted acetonitrile layer (1 mL) was evaporated to dryness under a stream of nitrogen at about 40 °C and reconstituted in 75 µL methanol and 425 µL water. The resulting extract was mixed for about 5 s using a vortex mixer and ultracentrifuged at 8500× g at room temperature for 10 min.
- “IAC”: Specific cleanup for AFLAs and OTA for sensitivity purposes when dealing with infant foods (e.g., infant cereals) and “difficult” matrices (e.g., spices, nuts, dried fruits). An aliquot of the acetonitrile layer (2 mL) was diluted in a PBS solution (25 mL), and the whole extract was applied onto IAC containing antibodies specific to AFLAs and OTA. The IAC was then washed with 20 mL water and the toxins finally eluted with methanol (3 × 800 µL). The eluate was evaporated to dryness under a stream of nitrogen at about 40 °C and reconstituted in 30 µL of methanol and 170 µL of water. The resulting extract was mixed for about 5 s using a vortex mixer and ultracentrifuged at 8500× g at room temperature for 10 min.
4.3. LC-MS/MS Analysis
4.4. Identification of Mycotoxins
4.5. Quantification
4.6. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paterson, R.R.M.; Lima, N. Toxicology of mycotoxins. In Molecular, Clinical and Environmental Toxicology: Volume 2: Clinical Toxicology; Luch, A., Ed.; Birkhäuser Basel: Basel, Switerland, 2010; pp. 31–63. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- European Union. COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. [Google Scholar]
- European Union. 2013/165/EU Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products Text with EEA relevance. Off. J. Eur. Union 2013, L 91, 12–15. [Google Scholar]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Schwab, W.; Yu, P. Natural Occurrence and Co-Contamination of Twelve Mycotoxins in Industry-Submitted Cool-Season Cereal Grains Grown under a Low Heat Unit Climate Condition. Toxins 2019, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Decision (EC) No 657/2002 of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union 2002, L 221, 8–36. [Google Scholar]
- Tittlemier, S.A.; Cramer, B.; Dall’Asta, C.; Iha, M.H.; Lattanzio, V.M.T.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; Stroka, J. Developments in mycotoxin analysis: an update for 2017–2018. World Mycotoxin J. 2019, 12, 3–29. [Google Scholar] [CrossRef]
- EN 16924:2017. Foodstuffs—Determination of Zearalenone in Edible Vegetable Oils by LC-FLD or LC-MS/MS; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- EN 16923:2017. Foodstuffs—Determination of T-2 Toxin and HT-2 Toxin in Cereals and Cereal Products for Infants and Young Children by LC-MS/MS after SPE Cleanup; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- European Union. MANDATE FOR STANDARDISATION ADDRESSED TO CEN FOR METHODS OF ANALYSIS FOR MYCOTOXINS IN FOOD. Available online: https://law.resource.org/pub/eu/mandates/m520.pdf (accessed on 19 September 2019).
- Pascale, M.; De Girolamo, A.; Lippolis, V.; Stroka, J.; Mol, H.G.J.; Lattanzio, V.M.T. Performance Evaluation of LC-MS Methods for Multimycotoxin Determination. J. AOAC Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Tessiot, S.; Bessaire, T.; Racault, L.; Fiorese, E.; Urbani, A.; Chan, W.C.; Cheng, P.; Mottier, P. Combining the quick, easy, cheap, effective, rugged and safe approach and clean-up by immunoaffinity column for the analysis of 15 mycotoxins by isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1337, 75–84. [Google Scholar] [CrossRef] [PubMed]
- EN 15662:2018. Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE. Modular QuEChERS-Method; European Committee for Standardization: Brussels, Belgium, 2018. [Google Scholar]
- Chan, W.-C.; Cheng, P.; Desmarchelier, A.; Fiorese, E.; Stephan, M. Mycotoxins in Foodstuffs by Isotope Dilution LC-MS/MS. In Proceedings of the World Mycotoxin Forum 8th Conference, Vienna, Austria, 10–12 November 2014. [Google Scholar]
- Bessaire, T. Prevention & Control of Mycotoxins at Nestlé—The Pivotal Role of Analytics. In Proceedings of the 12th International Fresenius Conference—Contaminants and Residues in Food, Cologne, Germany, 10 April 2019. [Google Scholar]
- ISO 17043:2010. Conformity Assessment—General Requirements for Proficiency Testing; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- AOAC International. Appendix D: Guidelines for Collaborative Study Procedures To Validate Characteristics of a Method of Analysis. J. AOAC Int. 1995, 78, 143A–160A. [Google Scholar]
- ISO 5725-2. Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- Zhang, K.; Schaab, M.R.; Southwood, G.; Tor, E.R.; Aston, L.S.; Song, W.; Eitzer, B.; Majumdar, S.; Lapainis, T.; Mai, H.; et al. A Collaborative Study: Determination of Mycotoxins in Corn, Peanut Butter, and Wheat Flour Using Stable Isotope Dilution Assay (SIDA) and Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). J. Agric. Food Chem. 2017, 65, 7138–7152. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Additi. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 819–835. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L 70, 12–34. [Google Scholar]
- Horwitz, W.; Kamps, L.R.; Boyer, K.W. Quality assurance in the analysis of foods and trace constituents. J. Assoc. Off. Anal. Chem. 1980, 63, 1344–1354. [Google Scholar] [PubMed]
- Thompson, M. Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst 2000, 125, 385–386. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Bibi, R.; Ciriaci, M.; Paoloni, A.; Pecorelli, I. Multimycotoxin Analysis by LC-MS/MS in Cereal Food and Feed: Comparison of Different Approaches for Extraction, Purification, and Calibration. J. AOAC Int. 2018, 101, 647–657. [Google Scholar] [CrossRef] [PubMed]
- European Union. Summary Report of the Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 17 September 2018. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/reg-com_toxic_20180917_sum.pdf?_cldee=bXZhbndlZWRlQGZubGkubmw%3d&recipientid=contact-2800eeaa6e2be8118127e0071b65ce91-45527089537b4f53a6f83c9233e6de1d&esid=3b6db41a-2660-e911-a96d-000d3ab490f3 (accessed on 30 September 2019).
- SANTE/12089/2016. Guidance Document on Identification of Mycotoxins in Food and Feed; European Commission Directorate General for Health and Food Safety: Brussels, Belgium, 2016. [Google Scholar]
- SANTE/11813/2017. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commission Directorate General for Health and Food Safety: Brussels, Belgium, 2017. [Google Scholar]


| Mycotoxin(s) | Document | Scope | Technique |
|---|---|---|---|
| AFB1 | AOAC 990.32 | Corn, roasted peanuts | ELISA |
| AFB1 | AOAC 2000.16 | Infant formula | HPLC-FLD (IAC) |
| AFB1 | EN 15851:2010 | Cereals, cereal based foods 1 | HPLC-FLD (IAC) |
| AFB1 | AOAC 978.15 | Egg | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 990.34 | Corn, cotton seed, peanuts, peanut butter | ELISA |
| AFB1, AFB2, AFG1, AFG2 | AOAC 991.31 | Corn, raw peanuts, peanut butter | HPLC-FLD (IAC) |
| AFB1, AFB2, AFG1, AFG2 | AOAC 2005.08 | Corn, raw peanuts, peanut butter | HPLC-FLD (IAC) |
| AFB1, AFB2, AFG1, AFG2 | AOAC 994.08 | Corn, almonds, nuts, peanuts, pistachio nuts | HPLC-FLD |
| AFB1, AFB2, AFG1, AFG2 | AOAC 968.22 | Peanuts and peanut products | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 970.45 | Peanuts and peanut products | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 972.27 | Soybeans | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 971.23 | Cocoa beans | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 971.24 | Coconut, copra | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 972.26 | Corn | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 993.17 | Corn, peanuts | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 970.46 | Green coffee | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 974.16 | Pistachio nuts | TLC |
| AFB1, AFB2, AFG1, AFG2 | AOAC 998.03 | Shelled peanuts | TLC |
| AFB1, Total AFLAs | AOAC 990.33 | Corn, peanut butter | HPLC-FLD |
| AFB1, Total AFLAs | ISO 16050:2003 | Cereals, nuts, oilseed products, dried fruits | HPLC-FLD |
| AFB1, Total AFLAs | AOAC 999.07 | Peanut butter, pistachio paste, fig paste, paprika | HPLC-FLD (IAC) |
| AFB1, Total AFLAs | EN 14123:2007 | Hazelnuts, peanuts, pistachios, figs, paprika | HPLC-FLD (IAC) |
| Total AFLAs | AOAC 991.45 | Peanut butter | ELISA |
| Total AFLAs | AOAC 993.16 | Corn | ELISA |
| Total AFLAs | AOAC 2013.05 | Olive oil, peanut oil, sesame oil | HPLC-FLD (IAC) |
| Total AFLAs | AOAC 975.36 | Corn, peanut, peanut butter, pistachio nuts | UV Lamp |
| Total AFLAs | AOAC 979.18 | Corn, raw and shelled peanuts | Visual fluorescence |
| AFM1 | ISO 14675:2003 | Milk products | ELISA |
| AFM1 | ISO 14501:2007 | Milk products | HPLC-FLD |
| AFM1 | AOAC 2000.08 | Liquid milk | HPLC-FLD (IAC) |
| AFM1 | ISO 14674:2005 | Milk products | TLC |
| AFM1 | AOAC 974.17 | Dairy products | TLC |
| AFM1 | AOAC 980.21 | Milk, cheese | TLC |
| AFM1 | AOAC 982.26 | Liver | TLC |
| AFM1, AFB1 | AOAC 982.24 | Liver | TLC |
| AFM1, AFB1 | AOAC 982.25 | Liver | TLC |
| AFM1, AFM2 2 | AOAC 986.16 | Liquid milk | HPLC-FLD |
| CIT 3 | EN 17203:2018 | Cereals, red yeast rice | LC-MS/MS |
| DON | AOAC 986.18 | Wheat | GC |
| DON | EN 15891:2010 | Cereals, cereal based foods 1 | HPLC-UV |
| DON | AOAC 986.17 | Wheat | TLC |
| FB1, FB2 | AOAC 2001.04 | Corn, corn flakes | HPLC-FLD (IAC) |
| FB1, FB2 | EN 16187:2015 | Maize based food 1 | HPLC-FLD (IAC) |
| FB1, FB2 | EN 14352:2004 | Maize based food | HPLC-FLD (IAC) |
| FB1, FB2, FB3 4 | AOAC 995.15 | Corn | HPLC-FLD |
| Total FBs | AOAC 2001.06 | Corn | ELISA |
| OTA | AOAC 991.44 | Barley, wheat, rye, corn | HPLC-FLD |
| OTA | ISO 15141:2018 | Cereals | HPLC-FLD |
| OTA | AOAC 2000.03 | Barley | HPLC-FLD (IAC) |
| OTA | AOAC 2000.09 | Roasted coffee | HPLC-FLD (IAC) |
| OTA | AOAC 2001.01 | Wines, beer | HPLC-FLD (IAC) |
| OTA | AOAC 2004.10 | Green coffee | HPLC-FLD (IAC) |
| OTA | EN 15835:2010 | Cereal based food | HPLC-FLD (IAC) |
| OTA | EN 15829:2010 | Dried fruits (currants, raisins, sultanas, figs) | HPLC-FLD (IAC) |
| OTA | EN 14133:2009 | Wine, beer | HPLC-FLD (IAC) |
| OTA | EN 14132:2009 | Barley, roasted coffee | HPLC-FLD (IAC) |
| OTA | ISO 15141:2018 | Cereals, cereal based products | HPLC-FLD (IAC) |
| OTA | AOAC 975.38 | Green coffee | TLC |
| OTA, OTB 5 | AOAC 973.37 | Barley | TLC |
| Patulin | ISO 8128-2:1993 | Apple juice and apple juice based products | HPLC-UV |
| Patulin | EN 15890:2010 | Fruit juice and fruit based purée 1 | HPLC-UV |
| Patulin | EN 14177:2003 | Clear and cloudy apple juice and puree | HPLC-UV |
| Patulin | AOAC 995.10 | Apple juice | HPLC-UV |
| Patulin | AOAC 2000.02 | Apple juice, apple puree | HPLC-UV |
| Patulin | ISO 8128-1:1993 | Apple juice and apple juice based products | TLC |
| Patulin | AOAC 974.18 | Apple juice | TLC |
| T-2, HT-2 | EN 16923:2017 | Cereals, cereal based foods 1 | LC-MS/MS |
| ZEN | AOAC 994.01 | Corn, wheat, feed | ELISA |
| ZEN | EN 15850:2010 | Cereals, cereal based foods 1 | HPLC-FLD (IAC) |
| ZEN | EN 16924:2017 | Edible vegetable oils | HPLC-FLD or |
| LC-MS/MS | |||
| ZEN | AOAC 976.22 | Corn | TLC |
| ZEN, α-ZEL 6 | AOAC 985.18 | Corn | HPLC-FLD |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | Assigned Value (µg/kg) | 5.4 | 11.4 | 11.3 | 6.54 | 8.2 | 2.48 | 4.86 | 2 | 4.58 | 0.26 | 0.51 | 0.0857 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 19 | |
| No. of Outliers | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | |
| No. of Accepted Results | 19 | 18 | 18 | 19 | 18 | 19 | 18 | 20 | 20 | 20 | 18 | 19 | |
| Mean (µg/kg) | 6.46 | 12.7 | 12.7 | 8.42 | 8.44 | 2.89 | 4.86 | 2.33 | 4.97 | 0.237 | 0.491 | 0.0848 | |
| SDr (µg/kg) | 0.32 | 0.53 | 0.47 | 0.71 | 0.3 | 0.14 | 0.29 | 0.45 | 0.39 | 0.013 | 0.028 | 0.0101 | |
| RSDr (%) | 5 | 4 | 4 | 8 | 4 | 5 | 6 | 19 | 8 | 5 | 6 | 12 | |
| SDR (µg/kg) | 0.67 | 1.15 | 1.31 | 1.22 | 0.6 | 0.3 | 0.69 | 0.54 | 0.57 | 0.031 | 0.036 | 0.0155 | |
| RSDR (%) | 10 | 9 | 10 | 15 | 7 | 10 | 14 | 23 | 12 | 13 | 7 | 18 | |
| Rec (%) | 120 | 111 | 113 | 129 | 103 | 116 | 100 | 116 | 109 | 91 | 96 | 99 | |
| HorRat Values | 0.5 | 0.4 | 0.5 | 0.7 | 0.3 | 0.5 | 0.6 | 1.1 | 0.5 | 0.6 | 0.3 | 0.8 | |
| AFB2 | Assigned Value (µg/kg) | 3.3 | 12.5 | 0.9 | 5.59 | 4.4 | 0.96 | n.d. | 1.9 | 2.01 | 0.25 | 0.5 | 0.0792 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 20 | 20 | - | 18 | 20 | 20 | 20 | 19 | |
| No. of Outliers | 2 | 2 | 0 | 2 | 1 | 1 | - | 1 | 0 | 0 | 1 | 2 | |
| No. of Accepted Results | 18 | 18 | 20 | 17 | 19 | 19 | - | 17 | 20 | 20 | 19 | 17 | |
| Mean (µg/kg) | 4.06 | 13.7 | 0.991 | 6.52 | 4.22 | 1.09 | - | 2.07 | 2.1 | 0.269 | 0.483 | 0.0778 | |
| SDr (µg/kg) | 0.12 | 0.71 | 0.062 | 0.46 | 0.17 | 0.05 | - | 0.15 | 0.26 | 0.022 | 0.022 | 0.0077 | |
| RSDr (%) | 3 | 5 | 6 | 7 | 4 | 4 | - | 7 | 12 | 8 | 5 | 10 | |
| SDR (µg/kg) | 0.22 | 0.91 | 0.115 | 0.84 | 0.36 | 0.09 | - | 0.25 | 0.24 | 0.027 | 0.031 | 0.0121 | |
| RSDR (%) | 5 | 7 | 12 | 13 | 9 | 8 | - | 12 | 11 | 10 | 6 | 16 | |
| Rec (%) | 123 | 109 | 110 | 117 | 96 | 113 | - | 109 | 105 | 108 | 97 | 98 | |
| HorRat Values | 0.2 | 0.3 | 0.5 | 0.6 | 0.4 | 0.4 | - | 0.5 | 0.5 | 0.5 | 0.3 | 0.7 | |
| AFG1 | Assigned Value (µg/kg) | 2.1 | 20.9 | 9.5 | 3.03 | 1.8 | 2.65 | n.d. | 1.7 | 7.77 | 0.2 | 0.5 | 0.0628 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 20 | 20 | - | 18 | 20 | 20 | 20 | 19 | |
| No. of Outliers | 1 | 1 | 1 | 2 | 0 | 0 | - | 1 | 0 | 1 | 0 | 3 | |
| No. of Accepted Results | 19 | 19 | 19 | 17 | 20 | 20 | - | 17 | 20 | 19 | 20 | 16 | |
| Mean (µg/kg) | 2.06 | 21.6 | 10.2 | 3.58 | 1.79 | 3.02 | - | 1.71 | 8.42 | 0.199 | 0.504 | 0.0613 | |
| SDr (µg/kg) | 0.094 | 0.75 | 0.49 | 0.26 | 0.093 | 0.24 | - | 0.17 | 0.65 | 0.027 | 0.018 | 0.0049 | |
| RSDr (%) | 5 | 3 | 5 | 7 | 5 | 8 | - | 10 | 8 | 13 | 4 | 8 | |
| SDR (µg/kg) | 0.15 | 1.55 | 0.79 | 0.35 | 0.15 | 0.27 | - | 0.24 | 1.1 | 0.023 | 0.034 | 0.0083 | |
| RSDR (%) | 7 | 7 | 8 | 10 | 8 | 9 | - | 14 | 13 | 11 | 7 | 14 | |
| Rec (%) | 98 | 103 | 107 | 118 | 99 | 114 | - | 101 | 108 | 100 | 101 | 98 | |
| HorRat Values | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | - | 0.6 | 0.6 | 0.5 | 0.3 | 0.6 | |
| AFG2 | Assigned Value (µg/kg) | 2.2 | 15 | 11.9 | 2.84 | 0.9 | 0.83 | n.d. | 3.7 | 5.79 | 0.31 | 0.52 | 0.052 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 19 | 19 | - | 19 | 20 | 20 | 20 | 15 | |
| No. of Outliers | 2 | 1 | 0 | 2 | 1 | 3 | - | 0 | 0 | 1 | 2 | 1 | |
| No. of Accepted Results | 18 | 19 | 20 | 17 | 18 | 16 | - | 19 | 20 | 19 | 18 | 14 | |
| Mean (µg/kg) | 2.32 | 16.4 | 11.3 | 3.46 | 0.814 | 0.885 | - | 3.13 | 6.14 | 0.304 | 0.488 | 0.052 | |
| SDr (µg/kg) | 0.1 | 1.01 | 0.65 | 0.33 | 0.059 | 0.055 | - | 0.48 | 0.56 | 0.041 | 0.045 | 0.0077 | |
| RSDr (%) | 4 | 6 | 6 | 9 | 7 | 6 | - | 15 | 9 | 14 | 9 | 15 | |
| SDR (µg/kg) | 0.19 | 1.85 | 0.97 | 0.37 | 0.133 | 0.072 | - | 0.66 | 1.05 | 0.048 | 0.043 | 0.0077 | |
| RSDR (%) | 8 | 11 | 9 | 11 | 16 | 8 | - | 21 | 17 | 16 | 9 | 15 | |
| Rec (%) | 105 | 109 | 95 | 122 | 90 | 107 | - | 84 | 106 | 98 | 94 | 100 | |
| HorRat Values | 0.4 | 0.5 | 0.4 | 0.5 | 0.7 | 0.4 | - | 1 | 0.8 | 0.7 | 0.4 | 0.7 | |
| Total AFLAs | Assigned Value (µg/kg) | 12.5 | 58.4 | 33.8 | 19.2 | 15.2 | 6.81 | 4.86 | 9.2 | 20.2 | 1.05 | 2.07 | 0.28 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 20 | 19 | 20 | 20 | 20 | 20 | 20 | 19 | |
| No. of Outliers | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | |
| No. of Accepted Results | 20 | 20 | 20 | 19 | 20 | 18 | 18 | 20 | 20 | 19 | 19 | 18 | |
| Mean (µg/kg) | 15 | 65.4 | 35.6 | 21.7 | 15.3 | 7.83 | 4.86 | 8.9 | 21.6 | 1 | 1.95 | 0.268 | |
| SDr (µg/kg) | 0.67 | 3.04 | 2.09 | 1.3 | 0.76 | 0.35 | 0.29 | 0.65 | 1.36 | 0.051 | 0.058 | 0.0305 | |
| RSDr (%) | 4 | 5 | 6 | 6 | 5 | 4 | 6 | 7 | 6 | 5 | 3 | 11 | |
| SDR (µg/kg) | 1.22 | 5.22 | 3.16 | 2.36 | 1.15 | 0.56 | 0.69 | 1.36 | 2.16 | 0.073 | 0.097 | 0.0351 | |
| RSDR (%) | 8 | 8 | 9 | 11 | 8 | 7 | 14 | 15 | 10 | 7 | 5 | 13 | |
| Rec (%) | 120 | 112 | 105 | 113 | 101 | 115 | 100 | 97 | 107 | 95 | 94 | 96 | |
| HorRat Values | 0.4 | 0.4 | 0.4 | 0.5 | 0.3 | 0.3 | 0.6 | 0.7 | 0.5 | 0.3 | 0.2 | 0.6 | |
| OTA | Assigned Value (µg/kg) | 16.9 | 7 | 12 | 17.2 | 1 | 9.05 | 3.46 | 4.1 | 2.2 | 0.52 | 0.51 | 0.448 |
| No. of Laboratories | 20 | 20 | 20 | 18 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| No. of Outliers | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | |
| No. of Accepted Results | 19 | 20 | 20 | 17 | 20 | 19 | 18 | 20 | 18 | 19 | 20 | 20 | |
| Mean (µg/kg) | 17.7 | 8.3 | 14.5 | 20.9 | 1.05 | 9.81 | 3.37 | 4.51 | 2.72 | 0.6 | 0.535 | 0.444 | |
| SDr (µg/kg) | 0.64 | 0.21 | 0.77 | 1.26 | 0.06 | 0.31 | 0.37 | 0.54 | 0.22 | 0.03 | 0.0345 | 0.0341 | |
| RSDr (%) | 4 | 3 | 5 | 6 | 5 | 3 | 11 | 12 | 8 | 5 | 6 | 8 | |
| SDR (µg/kg) | 2.73 | 1.27 | 1.91 | 4.16 | 0.16 | 1.33 | 0.43 | 1.27 | 0.44 | 0.069 | 0.06 | 0.056 | |
| RSDR (%) | 15 | 15 | 13 | 20 | 15 | 14 | 13 | 28 | 16 | 11 | 11 | 13 | |
| Rec (%) | 105 | 119 | 121 | 122 | 105 | 108 | 97 | 110 | 123 | 116 | 105 | 99 | |
| HorRat Values | 0.7 | 0.7 | 0.6 | 0.9 | 0.7 | 0.6 | 0.6 | 1.3 | 0.7 | 0.5 | 0.5 | 0.6 |
| S7 | S8 | S9 | S10 | S11 | S12 | ||
|---|---|---|---|---|---|---|---|
| ZEN | Assigned Value (µg/kg) | 5.4 | 11.4 | 11.3 | 6.54 | 8.2 | 2.48 |
| No. of Laboratories | 20 | 20 | 20 | 19 | 20 | 20 | |
| No. of Outliers | 1 | 2 | 2 | 0 | 2 | 1 | |
| No. of Accepted Results | 19 | 18 | 18 | 19 | 18 | 19 | |
| Mean (µg/kg) | 6.46 | 12.7 | 12.7 | 8.42 | 8.44 | 2.89 | |
| SDr (µg/kg) | 0.32 | 0.53 | 0.47 | 0.71 | 0.3 | 0.14 | |
| RSDr (%) | 5 | 4 | 4 | 8 | 4 | 5 | |
| SDR (µg/kg) | 0.67 | 1.15 | 1.31 | 1.22 | 0.6 | 0.3 | |
| RSDR (%) | 10 | 9 | 10 | 15 | 7 | 10 | |
| Rec (%) | 120 | 111 | 113 | 129 | 103 | 116 | |
| HorRat Values | 0.5 | 0.4 | 0.5 | 0.7 | 0.3 | 0.5 | |
| DON | Assigned Value (µg/kg) | 743 | 506 | 176 | 220 | 295 | 45.2 |
| No. of Laboratories | 20 | 20 | 20 | 18 | 20 | 20 | |
| No. of Outliers | 1 | 0 | 1 | 1 | 0 | 0 | |
| No. of Accepted Results | 19 | 20 | 19 | 17 | 20 | 20 | |
| Mean (µg/kg) | 739 | 527 | 195 | 220 | 269 | 43.7 | |
| SDr (µg/kg) | 22 | 41.5 | 12.3 | 10.4 | 18.9 | 3.3 | |
| RSDr (%) | 3 | 8 | 6 | 5 | 7 | 8 | |
| SDR (µg/kg) | 48.5 | 52.5 | 13.6 | 15.5 | 25.3 | 3.86 | |
| RSDR (%) | 7 | 10 | 7 | 7 | 9 | 9 | |
| Rec (%) | 99 | 104 | 111 | 100 | 91 | 97 | |
| HorRat Values | 0.4 | 0.6 | 0.3 | 0.3 | 0.5 | 0.4 | |
| T-2 | Assigned Value (µg/kg) | 57.9 | 43 | 10.3 | 15 | 23.3 | 11.3 |
| No. of Laboratories | 19 | 19 | 19 | 18 | 19 | 19 | |
| No. of Outliers | 1 | 0 | 0 | 0 | 0 | 2 | |
| No. of Accepted Results | 18 | 19 | 19 | 18 | 19 | 17 | |
| Mean (µg/kg) | 56.9 | 44.4 | 9.66 | 16.1 | 22.2 | 10.6 | |
| SDr (µg/kg) | 3.5 | 3.29 | 0.78 | 0.89 | 1.52 | 0.7 | |
| RSDr (%) | 6 | 7 | 8 | 6 | 7 | 7 | |
| SDR (µg/kg) | 3.78 | 5.98 | 2.15 | 1.54 | 2.2 | 0.87 | |
| RSDR (%) | 7 | 13 | 22 | 10 | 10 | 8 | |
| Rec (%) | 98 | 103 | 94 | 107 | 95 | 94 | |
| HorRat Values | 0.3 | 0.6 | 1 | 0.4 | 0.5 | 0.4 | |
| HT-2 | Assigned Value (µg/kg) | 81.8 | 27 | 28.6 | 19 | 14.5 | 9.5 |
| No. of Laboratories | 19 | 19 | 19 | 17 | 19 | 19 | |
| No. of Outliers | 0 | 0 | 0 | 1 | 0 | 0 | |
| No. of Accepted Results | 19 | 19 | 19 | 16 | 19 | 19 | |
| Mean (µg/kg) | 81.1 | 30.3 | 32.3 | 15.9 | 16.1 | 8.1 | |
| SDr (µg/kg) | 5.19 | 2.34 | 2.65 | 1.24 | 1.79 | 0.82 | |
| RSDr (%) | 6 | 8 | 8 | 8 | 11 | 10 | |
| SDR (µg/kg) | 10.2 | 4.67 | 4.65 | 1.8 | 2.88 | 1.03 | |
| RSDR (%) | 13 | 15 | 14 | 11 | 18 | 13 | |
| Rec (%) | 99 | 112 | 113 | 83 | 111 | 85 | |
| HorRat Values | 0.6 | 0.7 | 0.7 | 0.5 | 0.8 | 0.6 | |
| T-2 + HT-2 | Assigned Value (µg/kg) | 132 | 68 | 38.9 | 36 | 38.2 | 20.8 |
| No. of Laboratories | 19 | 19 | 19 | 17 | 19 | 19 | |
| No. of Outliers | 0 | 0 | 0 | 1 | 0 | 0 | |
| No. of Accepted Results | 19 | 19 | 19 | 16 | 19 | 19 | |
| Mean (µg/kg) | 137 | 74.7 | 42 | 31.8 | 38.4 | 18.7 | |
| SDr (µg/kg) | 6.49 | 4.43 | 2.89 | 1.98 | 2.83 | 1.17 | |
| RSDr (%) | 5 | 6 | 7 | 6 | 7 | 6 | |
| SDR (µg/kg) | 12.6 | 6.72 | 5.45 | 2.49 | 3.97 | 1.92 | |
| RSDR (%) | 9 | 9 | 13 | 8 | 10 | 10 | |
| Rec (%) | 104 | 110 | 108 | 88 | 100 | 90 | |
| HorRat Values | 132 | 68 | 38.9 | 36 | 38.2 | 20.8 | |
| FB1 | Assigned Value (µg/kg) | 275 | 4262 | n.d | 72 | 121 | 31.1 |
| No. of Laboratories | 20 | 18 | - | 20 | 20 | 20 | |
| No. of Outliers | 0 | 0 | - | 1 | 0 | 0 | |
| No. of Accepted Results | 20 | 18 | - | 19 | 20 | 20 | |
| Mean (µg/kg) | 291 | 4735 | - | 76.5 | 125 | 33.1 | |
| SDr (µg/kg) | 14.9 | 258 | - | 4.32 | 8.15 | 3.14 | |
| RSDr (%) | 5 | 5 | - | 6 | 7 | 9 | |
| SDR (µg/kg) | 35.2 | 541 | - | 11.9 | 18.6 | 5.53 | |
| RSDR (%) | 12 | 11 | - | 16 | 15 | 17 | |
| Rec (%) | 106 | 111 | - | 106 | 103 | 106 | |
| HorRat Values | 0.6 | 0.9 | - | 0.7 | 0.7 | 0.8 | |
| FB2 | Assigned Value (µg/kg) | 223 | 1299 | n.d | 72 | 130 | 44.2 |
| No. of Laboratories | 20 | 19 | - | 20 | 20 | 20 | |
| No. of Outlier | 0 | 0 | - | 2 | 2 | 2 | |
| No. of Accepted Results | 20 | 19 | - | 18 | 18 | 18 | |
| Mean (µg/kg) | 245 | 1500 | - | 74.8 | 134 | 41.1 | |
| SDr (µg/kg) | 10 | 72.2 | - | 5.46 | 7.45 | 4.17 | |
| RSDr (%) | 4 | 5 | - | 7 | 6 | 10 | |
| SDR (µg/kg) | 25 | 180 | - | 7.25 | 10 | 4.63 | |
| RSDR (%) | 10 | 12 | - | 10 | 7 | 11 | |
| Rec (%) | 110 | 115 | - | 104 | 103 | 93 | |
| HorRat Values | 0.5 | 0.8 | - | 0.4 | 0.3 | 0.5 | |
| Total FBs | Assigned Value (µg/kg) | 485 | 5528 | n.d. | 150 | 245 | 75.3 |
| No. of Laboratories | 20 | 18 | - | 20 | 20 | 20 | |
| No. of Outliers | 0 | 0 | - | 2 | 1 | 0 | |
| No. of Accepted Results | 20 | 18 | - | 18 | 19 | 20 | |
| Mean (µg/kg) | 536 | 6233 | - | 152 | 263 | 76 | |
| SDr (µg/kg) | 18.6 | 291 | - | 8.9 | 12.3 | 5.6 | |
| RSDr (%) | 3 | 5 | - | 6 | 5 | 7 | |
| SDR (µg/kg) | 51.5 | 588 | - | 16.1 | 26.7 | 11.3 | |
| RSDR (%) | 10 | 9 | - | 11 | 10 | 15 | |
| Rec (%) | 110 | 113 | - | 101 | 107 | 101 | |
| HorRat Values | 0.5 | 0.8 | - | 0.5 | 0.5 | 0.7 |
| S13 | S14 | ||
|---|---|---|---|
| AFM1 | Assigned Value (µg/kg) | 0.1121 | 0.0342 |
| No. of Laboratories | 20 | 16 | |
| No. of Outliers | 2 | 2 | |
| No. of Accepted Results | 18 | 14 | |
| Mean (µg/kg) | 0.127 | 0.0333 | |
| SDr (µg/kg) | 0.013 | 0.0073 | |
| RSDr (%) | 10 | 22 | |
| SDR (µg/kg) | 0.017 | 0.0087 | |
| RSDR (%) | 13 | 26 | |
| Rec (%) | 113 | 97 | |
| HorRat Values | 0.6 | 1.2 |
| Mycotoxins | ML (µg/kg) a | Lowest Validated Level (µg/kg) | RSDr (%) | RSDR (%) | Rec% |
|---|---|---|---|---|---|
| AFB1 | 0.1 | 0.084 | 12 | 18 | 99 |
| OTA | 0.5 | 0.44 | 8 | 13 | 99 |
| DON | 200 | 43.6 | 8 | 9 | 97 |
| ZEN | 20 | 8.8 | 8 | 13 | 92 |
| T-2 + HT-2 | 15 b | 18.6 | 6 | 10 | 90 |
| FBTOT | 200 | 76.2 | 7 | 15 | 101 |
| AFM1 | 0.025 c | 0.0117 | 10 | 13 | 113 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessaire, T.; Mujahid, C.; Mottier, P.; Desmarchelier, A. Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study. Toxins 2019, 11, 658. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110658
Bessaire T, Mujahid C, Mottier P, Desmarchelier A. Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study. Toxins. 2019; 11(11):658. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110658
Chicago/Turabian StyleBessaire, Thomas, Claudia Mujahid, Pascal Mottier, and Aurélien Desmarchelier. 2019. "Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study" Toxins 11, no. 11: 658. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11110658