Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics
Abstract
:1. Introduction: Venom Contents and Cellular Targets
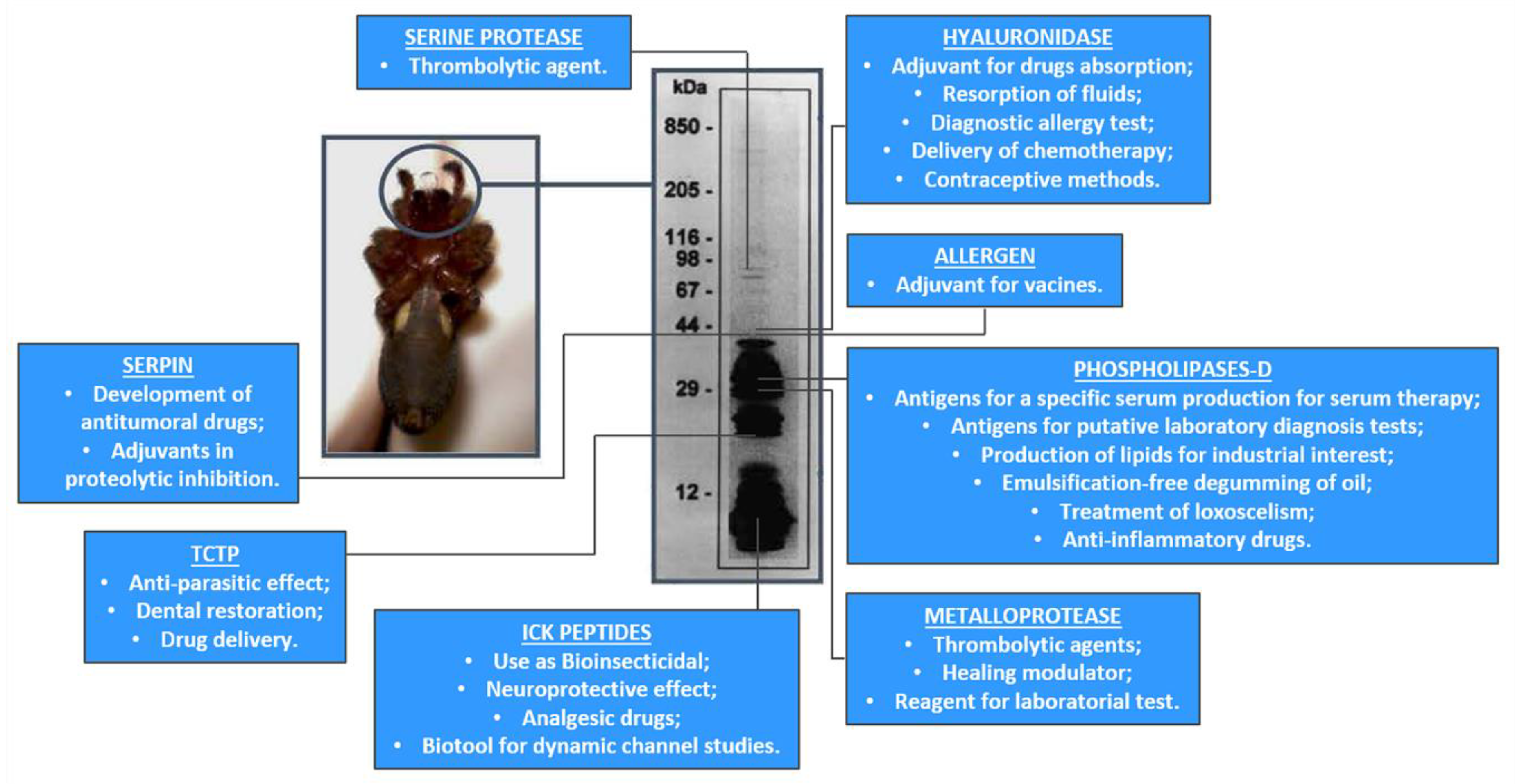
2. Recombinant Toxins: Biotools and Drug Targets
3. ICK Peptides: Analgesic Drug, Neuroprotective Effector and Bioinsecticide

4. Phospholipase-D: Treatment of Loxoscelism, Chemotherapy Drugs, and Anti-Inflammatory Drugs
5. Proteases: Matrix Modulator and Thrombolytic Agent
6. Serine Protease Inhibitors: Anti-Proliferative and Anti-Metastatic Activities, Adjuvants in the Proteolytic Inhibition and Agricultural Pest Regulators
7. Hyaluronidases: Adjuvant for Drug Absorption, Diagnostic Allergy Tests, Delivery of Chemotherapy and Contraceptive Molecules
8. TCTP: Antiparasitic Effect, Dental Restoration and Drug Delivery
9. Brown Spider Venom Toxins: New Immunotherapies
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 1992, 267, 7402–7405. [Google Scholar] [PubMed]
- Smith, C.G.; Vane, J.R. The discovery of captopril. FASEB J. 2003, 17, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Prommer, E. Ziconotide: A new option for refractory pain. Drugs Today 2006, 42, 369–378. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.H.; da Silveira, R.B.; Appel, M.H.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Brown spiders and loxoscelism. Toxicon 2004, 44, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Gremski, L.H.; Trevisan-Silva, D.; Ferrer, V.P.; Matsubara, F.H.; Meissner, G.O.; Wille, A.C.; Vuitika, L.; Dias-Lopes, C.; Ullah, A.; de Moraes, F.R.; et al. Recent advances in the understanding of brown spider venoms: From the biology of spiders to the molecular mechanisms of toxins. Toxicon 2014, 83, 91–120. [Google Scholar] [CrossRef]
- Ferrer, V.P.; de Mari, T.L.; Gremski, L.H.; Trevisan-Silva, D.; da Silveira, R.B.; Gremski, W.; Chaim, O.M.; Senff-Ribeiro, A.; Nader, H.B.; Veiga, S.S. A novel hyaluronidase from brown spider (Loxosceles intermedia) venom (Dietrich’s Hyaluronidase): From cloning to functional characterization. PLoS Negl. Trop. Dis. 2013, 7, e2206. [Google Scholar] [CrossRef] [PubMed]
- de Castro, C.S.; Silvestre, F.G.; Araujo, S.C.; Gabriel, M.Y.; Mangili, O.C.; Cruz, I.; Chávez-Olórtegui, C.; Kalapothakis, E. Identification and molecular cloning of insecticidal toxins from the venom of the brown spider Loxosceles intermedia. Toxicon 2004, 44, 273–280. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Bednaski, A.V.; Fischer, J.S.G.; Veiga, S.S.; Bandeira, N.; Guthals, A.; Marchini, F.K.; Leprevost, F.V.; Barbosa, V.C.; Senff-Ribeiro, A.; et al. A multi-protease, multi-dissociation, bottom-up-to-top-down proteomic view of the Loxosceles intermedia venom. Sci. Data 2017, 4, 170090. [Google Scholar] [CrossRef]
- Gremski, L.H.; da Silveira, R.B.; Chaim, O.M.; Probst, C.M.; Ferrer, V.P.; Nowatzki, J.; Weinschutz, H.C.; Madeira, H.M.; Gremski, W.; Nader, H.B.; et al. A novel expression profile of the Loxosceles intermedia spider venomous gland revealed by transcriptome analysis. Mol. Biosyst. 2010, 19, 2403–2416. [Google Scholar] [CrossRef]
- Sade, Y.B.; Boia-Ferreira, M.; Gremski, L.H.; da Silveira, R.B.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; Veiga, S.S. Molecular cloning, heterologous expression and functional characterization of a novel translationally-controlled tumor protein (TCTP) family member from Loxosceles intermedia (brown spider) venom. Int. J. Biochem. Cell Biol. 2012, 44, 170–177. [Google Scholar] [CrossRef]
- Ospedal, K.Z.; Appel, M.H.; Fillus-Neto, J.; Mangili, O.C.; Veiga, S.S.; Gremski, W. Histopathological findings in rabbits after experimental acute exposure to the Loxosceles intermedia (brown spider) venom. Int. J. Exp. Pathol. 2002, 83, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, K.C.; Lira, M.S.; Araujo, C.A.; Pareja-Santos, A.; Tavora, B.C.; Prezotto-Neto, J.P.; Kimura, L.F.; Lima, C.; Lopes-Ferreira, M.; Santoro, M.L. Inflammatory mediators generated at the site of inoculation of Loxosceles gaucho spider venom. Toxicon 2010, 56, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Lankford, H.A.; Warren, J.S. Loxosceles deserta spider venom induces the expression of vascular endothelial growth factor (VEGF) in keratinocytes. Inflammation 2000, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.S.; Zanetti, V.C.; Braz, A.; Mangili, O.C.; Gremski, W. Extracellular matrix molecules as targets for brown spider venom toxins. Braz. J. Med. Biol. Res. 2001, 34, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paludo, K.S.; Gremski, L.H.; Veiga, S.S.; Chaim, O.M.; Gremski, W.; de Freitas Buchi, D.; Nader, H.B.; Dietrich, C.P.; Franco, C.R. The effect of brown spider venom on endothelial cell morphology and adhesive structures. Toxicon 2006, 47, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.S.; Zanetti, V.C.; Franco, C.R.; Trindade, E.S.; Porcionatto, M.A.; Mangili, O.C.; Gremski, W.; Dietrich, C.P.; Nader, H.B. In vivo and in vitro cytotoxicity of brown spider venom for blood vessel endothelial cells. Thromb. Res. 2001, 102, 229–237. [Google Scholar] [CrossRef]
- Dragulev, B.; Bao, Y.; Ramos-Cerrillo, B.; Vazquez, H.; Olvera, A.; Stock, R.; Algaron, A.; Fox, J.W. Upregulation of IL-6, IL-8, CXCL1, and CXCL2 dominates gene expression in human fibroblast cells exposed to Loxosceles reclusa sphingomyelinase D: Insights into spider venom dermonecrosis. J. Investig. Dermatol. 2007, 127, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Chaim, O.M.; da Silveira, R.B.; Trevisan-Silva, D.; Ferrer, V.P.; Sade, Y.B.; Boia-Ferreira, M.; Gremski, L.H.; Gremski, W.; Senff-Ribeiro, A.; Takahashi, H.K.; et al. Phospholipase-D activity and inflammatory response induced by brown spider dermonecrotic toxin: Endothelial cell membrane phospholipids as targets for toxicity. Biochim. Biophys. Acta 2011, 1811, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves-Moreira, D.; Chaim, O.M.; Sade, Y.B.; Paludo, K.S.; Gremski, L.H.; Donatti, L.; de Moura, J.; Mangili, O.C.; Gremski, W.; da Silveira, R.B.; et al. Identification of a direct hemolytic effect dependent on the catalytic activity induced by phospholipase-D (dermonecrotic toxin) from brown spider venom. J. Cell. Biochem. 2009, 107, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Moreira, D.; Souza, F.N.; Fogaça, R.T.; Mangili, O.C.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; Veiga, S.S. The relationship between calcium and the metabolism of plasma membrane phospholipids in hemolysis induced by brown spider venom phospholipase-D toxin. J. Cell. Biochem. 2011, 112, 2529–2540. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Da Silva, M.S.; Billington, S.J.; Goncalves De Andrade, R.M.; Magnoli, F.C.; Songer, J.G.; Van Den Berg, C.W. Mechanism of induction of complement susceptibility of erythrocytes by spider and bacterial sphingomyelinases. Immunology 2002, 107, 93–101. [Google Scholar] [CrossRef]
- Appel, M.H.; da Silveira, R.B.; Chaim, O.M.; Paludo, K.S.; Silva, D.T.; Chaves-Moreira, D.; da Silva, P.H.; Mangili, O.C.; Senff-Ribeiro, A.; Gremski, W.; et al. Identification, cloning and functional characterization of a novel dermonecrotic toxin (phospholipase D) from brown spider (Loxosceles intermedia) venom. Biochim. Biophys. Acta 2008, 1780, 167–178. [Google Scholar] [CrossRef]
- da Silveira, R.B.; Pigozzo, R.B.; Chaim, O.M.; Appel, M.H.; Dreyfuss, J.L.; Toma, L.; Mangili, O.C.; Gremski, W.; Dietrich, C.P.; Nader, H.B.; et al. Molecular cloning and functional characterization of two isoforms of dermonecrotic toxin from Loxosceles intermedia (brown spider) venom gland. Biochimie 2006, 88, 1241–1253. [Google Scholar] [CrossRef]
- da Silveira, R.B.; Pigozzo, R.B.; Chaim, O.M.; Appel, M.H.; Silva, D.T.; Dreyfuss, J.L.; Toma, L.; Dietrich, C.P.; Nader, H.B.; Veiga, S.S.; et al. Two novel dermonecrotic toxins LiRecDT4 and LiRecDT5 from brown spider (Loxosceles intermedia) venom: From cloning to functional characterization. Biochimie 2007, 89, 289–300. [Google Scholar] [CrossRef]
- Tavares, F.L.; Peichoto, M.E.; Rangel Dde, M.; Barbaro, K.C.; Cirillo, M.C.; Santoro, M.L.; Sano-Martins, I.S. Loxosceles gaucho spider venom and its sphingomyelinase fraction trigger the main functions of human and rabbit platelets. Hum. Exp. Toxicol. 2011, 30, 1567–1574. [Google Scholar] [CrossRef]
- da Silva, P.H.; Hashimoto, Y.; dos Santos, F.A.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Hematological cell findings in bone marrow and peripheral blood of rabbits after experimental acute exposure to Loxosceles intermedia (brown spider) venom. Toxicon 2003, 42, 155–161. [Google Scholar] [CrossRef]
- Chaim, O.M.; Sade, Y.B.; da Silveira, R.B.; Toma, L.; Kalapothakis, E.; Chavez-Olortegui, C.; Mangili, O.C.; Gremski, W.; von Dietrich, C.P.; Nader, H.B.; et al. Brown spider dermonecrotic toxin directly induces nephrotoxicity. Toxicol. Appl. Pharmacol. 2006, 211, 64–77. [Google Scholar] [CrossRef]
- Kusma, J.; Chaim, O.M.; Wille, A.C.; Ferrer, V.P.; Sade, Y.B.; Donatti, L.; Gremski, W.; Mangili, O.C.; Veiga, S.S. Nephrotoxicity caused by brown spider venom phospholipase-D (dermonecrotic toxin) depends on catalytic activity. Biochimie 2008, 90, 1722–1736. [Google Scholar] [CrossRef]
- Feitosa, L.; Gremski, W.; Veiga, S.S.; Elias, M.C.; Graner, E.; Mangili, O.C.; Brentani, R.R. Detection and characterization of metalloproteinases with gelatinolytic, fibronectinolytic and fibrinogenolytic activities in brown spider (Loxosceles intermedia) venom. Toxicon 1998, 36, 1039–1051. [Google Scholar] [CrossRef]
- da Silveira, R.B.; Filho, J.F.S.; Mangili, O.C.; Veiga, S.S.; Gremski, W.; Nader, H.B. Identification of proteases in the extract of venom glands from brown spider. Toxicon 2002, 40, 815–822. [Google Scholar] [CrossRef]
- Senff-Ribeiro, A.; Henrique da Silva, P.; Chaim, O.M.; Gremski, L.H.; Paludo, K.S.; da Silveira, R.B.; Gremski, W.; Mangili, O.C.; Veiga, S.S. Biotechnological applications of brown spider (Loxosceles genus) venom toxins. Biotechnol. Adv. 2008, 26, 210–218. [Google Scholar] [CrossRef]
- Fernandes-Pedrosa, F.; Junqueira de Azevedo, I.L.; Goncalves-de-Andrade, R.M.; van den Berg, C.W.; Ramos, C.R.; Ho, P.L.; Tambourgi, D.V. Molecular cloning and expression of a functional dermonecrotic and haemolytic factor from Loxosceles laeta venom. Biochem. Biophys. Res. Commun. 2002, 298, 638–645. [Google Scholar] [CrossRef]
- Vuitika, L.; Gremski, L.H.; Belisario-Ferrari, M.R.; Chaves-Moreira, D.; Ferrer, V.P.; Senff-Ribeiro, A.; Chaim, O.M.; Veiga, S.S. Brown spider phospholipase-D containing a conservative mutation (D233E) in the catalytic site: Identification and functional characterization. J. Cell. Biochem. 2013, 114, 2479–2492. [Google Scholar] [CrossRef]
- Lee, S.; Lynch, K.R. Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA). Biochem. J. 2005, 391 Pt 2, 317–323. [Google Scholar] [CrossRef]
- Silvestre, F.G.; de Castro, C.S.; de Moura, J.F.; Giusta, M.S.; De Maria, M.; Alvares, E.S.; Lobato, F.C.; Assis, R.A.; Goncalves, L.A.; Gubert, I.C.; et al. Characterization of the venom from the Brazilian Brown Spider Loxosceles similis Moenkhaus, 1898 (Araneae, Sicariidae). Toxicon 2005, 46, 927–936. [Google Scholar] [CrossRef]
- Ramos-Cerrillo, B.; Olvera, A.; Odell, G.V.; Zamudio, F.; Paniagua-Solis, J.; Alagon, A.; Stock, R.P. Genetic and enzymatic characterization of sphingomyelinase D isoforms from the North American fiddleback spiders Loxosceles boneti and Loxosceles reclusa. Toxicon 2004, 44, 507–514. [Google Scholar] [CrossRef]
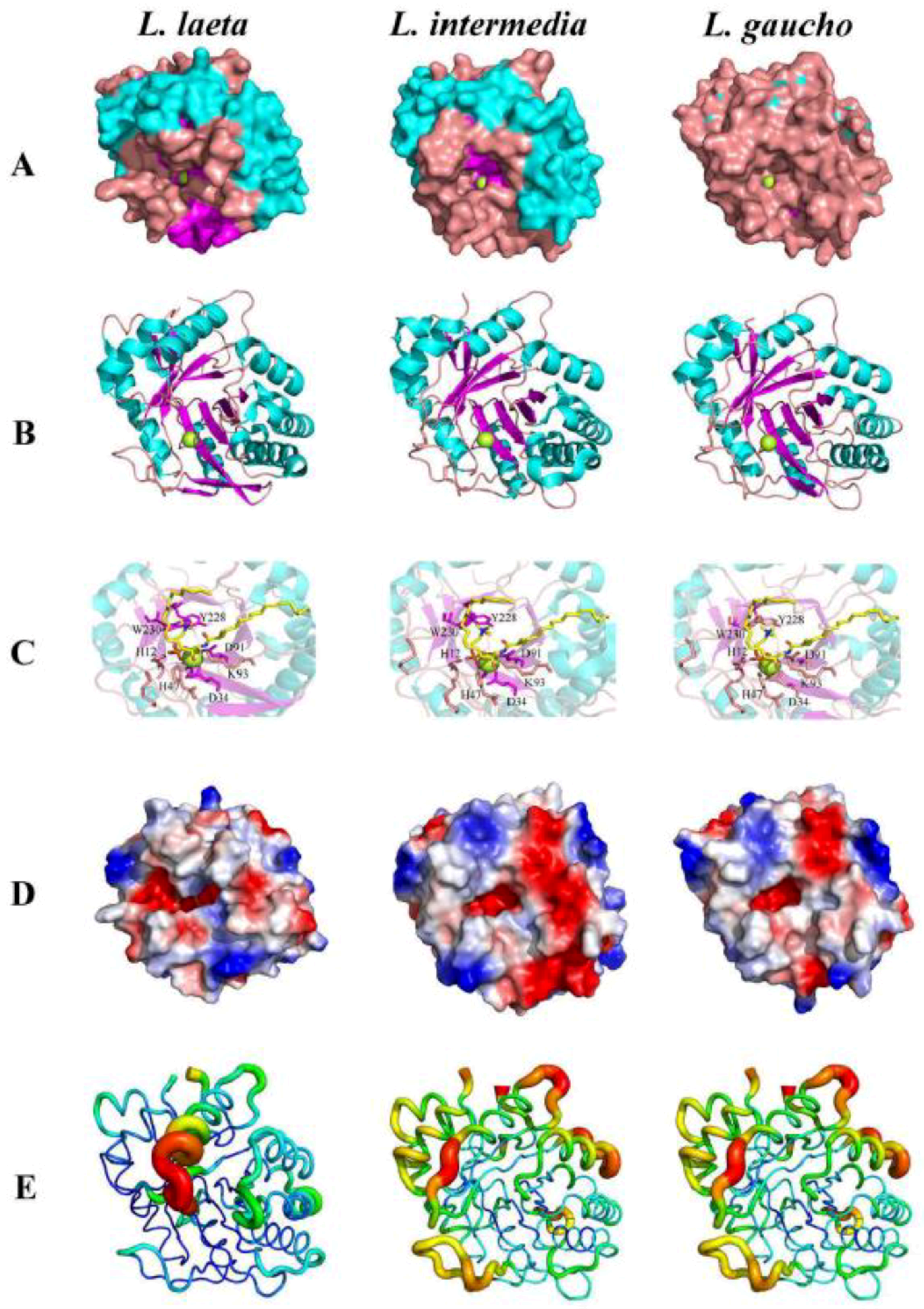
- Coronado, M.A.; Ullah, A.; da Silva, L.S.; Chaves-Moreira, D.; Vuitika, L.; Chaim, O.M.; Veiga, S.S.; Chahine, J.; Murakami, M.T.; Arni, R.K. Structural Insights into Substrate Binding of Brown Spider Venom Class II Phospholipases D. Curr. Protein Pept. Sci. 2015, 16, 768–774. [Google Scholar] [CrossRef]
- de Giuseppe, P.O.; Ullah, A.; Trevisan-Silva, D.; Gremski, L.H.; Wille, A.C.; Chaves Moreira, D.; Ribeiro, A.S.; Chaim, O.M.; Murakami, M.T.; Veiga, S.S.; et al. Structure of a novel class II phospholipase D: Catalytic cleft is modified by a disulphide bridge. Biochem. Biophys. Res. Commun. 2011, 409, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.T.; Fernandes-Pedrosa, M.F.; de Andrade, S.A.; Gabdoulkhakov, A.; Betzel, C.; Tambourgi, D.V.; Arni, R.K. Structural insights into the catalytic mechanism of sphingomyelinases D and evolutionary relationship to glycerophosphodiester phosphodiesterases. Biochem. Biophys. Res. Commun. 2006, 342, 323–329. [Google Scholar] [CrossRef]
- Murakami, M.T.; Fernandes-Pedrosa, M.F.; Tambourgi, D.V.; Arni, R.K. Structural basis for metal ion coordination and the catalytic mechanism of sphingomyelinases D. J. Biol. Chem. 2005, 280, 13658–13664. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Bednaski, A.V.; Gremski, L.H.; Chaim, O.M.; Veiga, S.S.; Senff-Ribeiro, A. Differential metalloprotease content and activity of three Loxosceles spider venoms revealed using two-dimensional electrophoresis approaches. Toxicon 2013, 76, 11–22. [Google Scholar] [CrossRef]
- da Silveira, R.B.; Wille, A.C.; Chaim, O.M.; Appel, M.H.; Silva, D.T.; Franco, C.R.; Toma, L.; Mangili, O.C.; Gremski, W.; Dietrich, C.P.; et al. Identification, cloning, expression and functional characterization of an astacin-like metalloprotease toxin from Loxosceles intermedia (brown spider) venom. Biochem. J. 2007, 406, 355–363. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Gremski, L.H.; Chaim, O.M.; da Silveira, R.B.; Meissner, G.O.; Mangili, O.C.; Barbaro, K.C.; Gremski, W.; Veiga, S.S.; Senff-Ribeiro, A. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 2010, 92, 21–32. [Google Scholar] [CrossRef]
- Matsubara, F.H.; Gremski, L.H.; Meissner, G.O.; Constantino Lopes, E.S.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; Veiga, S.S. A novel ICK peptide from the Loxosceles intermedia (brown spider) venom gland: Cloning, heterologous expression and immunological crossreactivity approaches. Toxicon 2013, 71, 147–158. [Google Scholar] [CrossRef]
- Lajoie, D.M.; Zobel-Thropp, P.A.; Kumirov, V.K.; Bandarian, V.; Binford, G.J.; Cordes, M.H. Phospholipase D toxins of brown spider venom convert lysophosphatidylcholine and sphingomyelin to cyclic phosphates. PLoS ONE 2013, 8, e72372. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Caporrino, M.C.; Della-Casa, M.S.; Kimura, L.F.; Prezotto-Neto, J.P.; Fukuda, D.A.; Portes-Junior, J.A.; Neves-Ferreira, A.G.; Santoro, M.L.; Barbaro, K.C. Cloning, expression and characterization of a phospholipase D from Loxosceles gaucho venom gland. Biochimie 2013, 95, 1773–1783. [Google Scholar] [CrossRef]
- De Bona, E.; (Federal University of Paraná, Curitiba, Paraná, Brazil). Personal communication, 2019.
- Da Justa, H.C.; (Federal University of Paraná, Curitiba, Paraná, Brazil). Personal communication, 2019.
- Norton, R.S.; Pallaghy, P.K. The cystine knot structure of ion channel toxins and related polypeptides. Toxicon 1998, 36, 1573–1583. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef]
- Herzig, V.; King, G.F. The Cystine Knot Is Responsible for the Exceptional Stability of the Insecticidal Spider Toxin ω-Hexatoxin-Hv1a. Toxins 2015, 7, 4366–4380. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.R.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Identification and Characterization of ProTx-III [mu-TRTX-Tp1a], a New Voltage-Gated Sodium Channel Inhibitor from Venom of the Tarantula Thrixopelma pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef]
- Zimmermann, L.; Morado-Diaz, C.J.; Davis-Lopez de Carrizosa, M.A.; de la Cruz, R.R.; May, P.J.; Streicher, J.; Pastor, Á.M.; Blumer, R. Axons giving rise to the palisade endings of feline extraocular muscles display motor features. J. Neurosci. 2013, 33, 2784–2793. [Google Scholar] [CrossRef]
- Klint, J.K.; Smith, J.J.; Vetter, I.; Rupasinghe, D.B.; Er, S.Y.; Senff, S.; Herzig, V.; Mobli, M.; Lewis, R.J.; Bosmans, F.; et al. Seven novel modulators of the analgesic target NaV 1.7 uncovered using a high-throughput venom-based discovery approach. Br. J. Pharmacol. 2015, 172, 2445–2458. [Google Scholar] [CrossRef]
- Netirojjanakul, C.; Miranda, L.P. Progress and challenges in the optimization of toxin peptides for development as pain therapeutics. Curr. Opin. Chem. Biol. 2017, 38, 70–79. [Google Scholar] [CrossRef]
- Baron, A.; Diochot, S.; Salinas, M.; Deval, E.; Noel, J.; Lingueglia, E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon 2013, 75, 187–204. [Google Scholar] [CrossRef]
- Mazzuca, M.; Heurteaux, C.; Alloui, A.; Diochot, S.; Baron, A.; Voilley, N.; Blondeau, N.; Escoubas, P.; Gélot, A.; Cupo, A.; et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat. Neurosci. 2007, 10, 943–945. [Google Scholar] [CrossRef]
- Pignataro, G.; Simon, R.P.; Xiong, Z.G. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 2007, 130 Pt 1, 151–158. [Google Scholar] [CrossRef]
- Yang, M.J.; Lin, W.Y.; Lu, K.H.; Tu, W.C. Evaluating antioxidative activities of amino acid substitutions on mastoparan-B. Peptides 2011, 32, 2037–2043. [Google Scholar] [CrossRef]
- Nunes, K.P.; Torres, F.S.; Borges, M.H.; Matavel, A.; Pimenta, A.M.C.; De Lima, M.E. New insights on arthropod toxins that potentiate erectile function. Toxicon 2013, 69, 152–159. [Google Scholar] [CrossRef]
- Ravelli, K.G.; Ramos, A.T.; Gonçalves, L.B.; Magnoli, F.C.; Troncone, L.R.P. Phoneutria nigriventer spider toxin Tx2-6 induces priapism in mice even after cavernosal denervation. Toxicon 2017, 130, 29–34. [Google Scholar] [CrossRef]
- Nunes-Silva, C.; Nunes, K.P.; Torres, F.S.; Cassoli, J.S.; Santos, D.M.; Almeida, F.D.M.; Matavel, A.; Cruz, J.S.; Santos-Miranda, A.; Nunes, A.D.; et al. PnPP-19, a Synthetic and Nontoxic Peptide Designed from a Phoneutria nigriventer Toxin, Potentiates Erectile Function via NO/cGMP. J. Urol. 2015, 194, 1481–1490. [Google Scholar] [CrossRef]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef]
- Matsubara, F.H.; Meissner, G.O.; Herzig, V.; Justa, H.C.; Dias, B.C.L.; Trevisan-Silva, D.; Gremski, L.H.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; et al. Insecticidal activity of a recombinant knottin peptide from Loxosceles intermedia venom and recognition of these peptides as conserved family in the genus. Insect Mol. Biol. 2017, 26, 25–34. [Google Scholar] [CrossRef]
- Stock, R.P.; Brewer, J.; Wagner, K.; Ramos-Cerrillo, B.; Duelund, L.; Jernshoj, K.D.; Olsen, L.F.; Bagatolli, L.A. Sphingomyelinase D activity in model membranes: Structural effects of in situ generation of ceramide-1-phosphate. PLoS ONE 2012, 7, e36003. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; Frederiks, F.; Giepmans, B.N.; Pedrosa, M.F.; Billington, S.J.; Jost, B.H.; Tambourgi, D.V.; Moolenaar, W.H. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 10833–10836. [Google Scholar] [CrossRef]
- Kalapothakis, E.; Chatzaki, M.; Goncalves-Dornelas, H.; de Castro, C.S.; Silvestre, F.G.; Laborne, F.V.; De Moura, J.F.; Veiga, S.S.; Chavez-Olortegui, C.; Granier, C.; et al. The Loxtox protein family in Loxosceles intermedia (Mello-Leitao) venom. Toxicon 2007, 50, 938–946. [Google Scholar] [CrossRef]
- Wille, A.C.; Chaves-Moreira, D.; Trevisan-Silva, D.; Magnoni, M.G.; Boia-Ferreira, M.; Gremski, L.H.; Gremski, W.; Chaim, O.M.; Senff-Ribeiro, A.; Veiga, S.S. Modulation of membrane phospholipids, the cytosolic calcium influx and cell proliferation following treatment of B16-F10 cells with recombinant phospholipase-D from Loxosceles intermedia (brown spider) venom. Toxicon 2013, 67, 17–30. [Google Scholar] [CrossRef]
- Machado, L.F.; Laugesen, S.; Botelho, E.D.; Ricart, C.A.; Fontes, W.; Barbaro, K.C.; Roepstorff, P.; Sousa, M.V. Proteome analysis of brown spider venom: Identification of loxnecrogin isoforms in Loxosceles gaucho venom. Proteomics 2005, 5, 2167–2176. [Google Scholar] [CrossRef]
- Vuitika, L.; Chaves-Moreira, D.; Caruso, I.; Lima, M.A.; Matsubara, F.H.; Murakami, M.T.; Takahashi, H.K.; Toledo, M.S.; Coronado, M.A.; Nader, H.B.; et al. Active site mapping of Loxosceles phospholipases D: Biochemical and biological features. Biochim. Biophys. Acta 2016, 1861, 970–979. [Google Scholar] [CrossRef]
- Chaves-Moreira, D.; Moraes, F.; Caruso, I.; Chaim, O.M.; Senff-Ribeiro, A.; Sussuchi, L.; Chahine, J.; Arni, R.K.; Veiga, S.S. Potential implications for drug design against phospholipase-D from Brown spider venom. J. Cell. Biochem. 2017, 118, 726–738. [Google Scholar] [CrossRef]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, functionality, and chemical modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef]
- Issuree, P.D.; Pushparaj, P.N.; Pervaiz, S.; Melendez, A.J. Resveratrol attenuates C5ainduced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009, 23, 2412–2424. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Veiga, S.S.; da Silveira, R.B.; Dreyfuss, J.L.; Haoach, J.; Pereira, A.M.; Mangili, O.C.; Gremski, W. Identification of high molecular weight serine-proteases in Loxosceles intermedia (brown spider) venom. Toxicon 2000, 38, 825–839. [Google Scholar] [CrossRef]
- Veiga, S.S.; Feitosa, L.; dos Santos, V.L.; de Souza, G.A.; Ribeiro, A.S.; Mangili, O.C.; Porcionatto, M.A.; Nader, H.B.; Dietrich, C.P.; Brentani, R.R.; et al. Effect of brown spider venom on basement membrane structures. Histochem. J. 2000, 32, 397–408. [Google Scholar] [CrossRef]
- Young, A.R.; Pincus, S.J. Comparison of enzymatic activity from three species of necrotising arachnids in Australia: Loxosceles rufescens, Badumna insignis and Lampona cylindrata. Toxicon 2001, 39, 391–400. [Google Scholar] [CrossRef]
- Fernandes-Pedrosa, F.; Junqueira-de-Azevedo, L.; Goncalves-de-Andrade, R.M.; Kobashi, L.S.; Almeida, D.D.; Ho, P.L.; ambourgi, D.V. Transcriptome analysis of Loxosceles laeta (Araneae, Sicariidae) spider venomous gland using expressed sequence tags. BMC Genom. 2008, 9, 279. [Google Scholar] [CrossRef]
- Chaim, O.M.; Trevisan-Silva, D.; Chaves-Moreira, D.; Wille, A.C.; Ferrer, V.P.; Matsubara, F.H.; Mangili, O.C.; Silveira, R.B.; Gremski, L.H.; Gremski, W.; et al. Brown spider (Loxosceles genus) venom toxins: Tools for biological purposes. Toxins 2011, 3, 309–344. [Google Scholar] [CrossRef]
- Sawant, R.; Nagendran, S. Protease: An enzyme with multiple industrial applications. J. Pharm. Pharm. Sci. 2014, 3, 568–579. [Google Scholar]
- Otlewski, J.; Krowarsch, D.; Apostoluk, W. Protein inhibitors of serine proteinases. Acta Biochim. Pol. 1999, 46, 531–565. [Google Scholar]
- Krowarsch, D.; Cierpicki, T.; Jelen, F.; Otlewski, J. Canonical protein inhibitors of serine proteases. Cell. Mol. Life Sci. 2003, 60, 2427–2444. [Google Scholar] [CrossRef]
- Huntington, J.A. Serpin structure, function and dysfunction. J Thromb. Haemost. 2011, 9, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Iaccarino, L.; Ghirardello, A.; Bassi, N.; Pontisso, P.; Punzi, L.; Shoenfeld, Y.; Doria, A. Serpins, immunity and autoimmunity: Old molecules, new functions. Clin. Rev. Allergy Immunol. 2013, 45, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Sanrattana, W.; Maas, C.; de Maat, S. SERPINs-From Trap to Treatment. Front. Med. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. Serine protease inhibitors (SERPINS): Where mechanism meets medicine. Nat. Med. 1996, 2, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Lomas, D.A. The molecular aetiology of the serpinopathies. Int. J. Biochem. Cell Biol. 2008, 40, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Lysvand, H.; Helland, R.; Hagen, L.; Slupphaug, G.; Iversen, O.J. Psoriasis pathogenesis–Pso p27 constitutes a compact structure forming large aggregates. Biochem. Biophys. Rep. 2015, 2, 132–136. [Google Scholar] [CrossRef]
- Lucas, A.; Yaron, J.R.; Zhang, L.; Macaulay, C.; McFadden, G. Serpins: Development for Therapeutic Applications. In Serpins Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1826, pp. 255–265. [Google Scholar]
- Yuan, C.H.; He, Q.Y.; Peng, K.; Diao, J.B.; Jiang, L.P.; Tang, X.; Liang, S.P. Discovery of a distinct superfamily of Kunitz-type toxin (KTT) from tarantulas. PLoS ONE 2008, 3, e3414. [Google Scholar] [CrossRef]
- Borges, M.H.; Figueiredo, S.G.; Leprevost, F.V.; De Lima, M.E.; Cordeiro, M.D.N.; Diniz, M.R.; Yates, J.R. Venomous extract protein profile of Brazilian tarantula Grammostola iheringi: Searching for potential biotechnological applications. J. Proteom. 2016, 136, 35–47. [Google Scholar] [CrossRef]
- Zupunski, V.Z.; Kordis, D.; Gubensek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Mulenga, A.; Khumthong, R.; Chalaire, K.C. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genom. 2009, 10, 217. [Google Scholar] [CrossRef]
- Meekins, D.A.; Kanost, M.R.; Michel, A. Serpins in arthropod biology. Semin. Cell Dev. Biol. 2017, 62, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, A.; Kim, T.; Ibelli, A.M. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 2013, 22, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Tirloni, L.; Radulovic, Z.; Lewis, L.; Bakshi, M.; Hill, C.; da Silva Vaz, I., Jr.; Logullo, C.; Termignoni, C.; Mulenga, A. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 2015, 45, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Chmelar, J.; Oliveira, C.J.; Rezacova, P.; Francischetti, I.M.; Kovarova, Z.; Pejler, G.; Kopacek, P.; Ribeiro, J.M.; Mares, M.; Kopecky, J.; et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 2011, 117, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle Lucca, J.J.; Li, Y.; Simovic, M.; Pusateri, A.E.; Falabella, M.; Dubick, M.A.; Tsokos, G.C. Effects of C1 Inhibitor on Tissue Damage in a Porcine Model of Controlled Hemorrhage. Shock 2012, 38, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, R.W.; Flores, D.N.; De La Cadena, R.A.; Scott, C.F.; Cousens, L.; Barr, P.J.; Hoffman, I.B.; Kueppers, F.; Fisher, D.; Idell, S.; et al. Recombinant alpha 1-antitrypsin Pittsburgh attenuates experimental gram-negative septicemia. Am. J. Pathol. 1988, 130, 418–426. [Google Scholar] [PubMed]
- Schaefer, J.S.; Zhang, M. Hypoxia effects: Implications for maspin regulation of the uPA/uPAR complex. Cancer Biol. Ther. 2005, 4, 1033–1035. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.M.; Dzinic, S.H.; Matta, M.J.; Dean, I.; Saker, L.; Sheng, S. The Opportunity of Precision Medicine for Breast Cancer with Context-Sensitive Tumor Suppressor Maspin. J. Cell. Biochem. 2017, 118, 1639–1647. [Google Scholar] [CrossRef]
- Katsukawa, M.; Ohsawa, S.; Zhang, L.; Yan, Y.; Igaki, T. Serpin Facilitates Tumor-Suppressive Cell Competition by Blocking Toll-Mediated Yki Activation in Drosophila. Curr. Biol. 2018, 28, 1756–1767. [Google Scholar] [CrossRef]
- Mika, A.; Reynolds, S.L.; Mohlin, F.C.; Willis, C.; Swe, P.M.; Pickering, D.A.; Halilovic, V.; Wijeyewickrema, L.C.; Pike, R.N.; Blom, A.M.; et al. Novel scabies mite serpins inhibit the three pathways of the human complement system. PLoS ONE 2012, 7, e40489. [Google Scholar] [CrossRef]
- Christeller, J.; Laing, W. Plant serine proteinase inhibitors. Prot. Pept. Lett. 2005, 12, 439–447. [Google Scholar] [CrossRef]
- Ribeiro, R.O.; Chaim, O.M.; da Silveira, R.B.; Gremski, L.H.; Sade, Y.B.; Paludo, K.S.; Senff-Ribeiro, A.; de Moura, J.; Chávez-Olórtegui, C.; Gremski, W.; et al. Biological and structural comparison of recombinant phospholipase D toxins from Loxosceles intermedia (brown spider) venom. Toxicon 2007, 50, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 2013, 62, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.M. Potential role of histamine releasing factor (HRF) as a therapeutic target for treating asthma and allergy. J. Asthma Allergy 2012, 5, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulenga, A.; Azad, A.F. The molecular and biological analysis of ixodid ticks histamine release factors. Exp. Appl. Acarol. 2005, 37, 215–229. [Google Scholar] [CrossRef]
- Buch, D.R.; Souza, F.N.; Meissner, G.O.; Morgon, A.M.; Gremski, L.H.; Ferrer, V.P.; Trevisan-Silva, D.; Matsubara, F.H.; Boia-Ferreira, M.; Sade, Y.B.; et al. Brown spider (Loxosceles genus) venom toxins: Evaluation of biological conservation by immune cross-reactivity. Toxicon 2015, 108, 154–166. [Google Scholar] [CrossRef]
- Kimura, T.; Ono, S.; Kubo, T. Molecular Cloning and Sequence Analysis of the cDNAs Encoding Toxin-Like Peptides from the Venom Glands of Tarantula Grammostola rosea. Int. J. Pept. 2012, 731293. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Correa, S.M.; Garb, J.E.; Binford, G.J. Spit and venom from scytodes spiders: A diverse and distinct cocktail. J. Proteome Res. 2014, 13, 817–835. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Wanichpakorn, S.; Kedjarune-Leggat, U. Translationally controlled tumor protein supplemented chitosan modified glass ionomer cement promotes osteoblast proliferation and function. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.J.; Van, T.T.; MacDonald, S.M.; Meshnick, S.R.; Fernley, R.T.; Macreadie, I.G.; Smooker, P.M. Immunization of mice with Plasmodium TCTP delays establishment of Plasmodium infection. Parasite Immunol. 2015, 37, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lan, J.; Wu, X.; Yang, D.; Zhang, Z.; Nie, H.; Hou, R.; Zhang, R.; Zheng, W.; Xie, Y.; et al. Expression of translationally controlled tumor protein (TCTP) gene of Dirofilaria immitis guided by transcriptomic screening. Korean J. Parasitol. 2014, 52, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bommer, U. Cellular Function and Regulation of the Translationally Controlled Tumour Protein TCTP. Open Allergy J. 2012, 5, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Kashiwakura, J.C.; Ando, T.; Matsumoto, K.; Kimura, M.; Kitaura, J.; Matho, M.H.; Zajonc, D.M.; Ozeki, T.; Ra, C.; MacDonald, S.M.; et al. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J. Clin. Investig. 2011, 122, 218–228. [Google Scholar] [CrossRef]
- Bae, H.D.; Lee, K. On employing a translationally controlled tumor protein-derived protein transduction domain analog for transmucosal delivery of drugs. J. Control. Release 2013, 170, 358–364. [Google Scholar] [CrossRef]
- Maeng, J.; Kim, H.Y.; Shin, D.H.; Lee, K. Transduction of translationally controlled tumor protein employing TCTP-derived protein transduction domain. Anal. Biochem. 2012, 435, 47–53. [Google Scholar] [CrossRef]
- Wanachottrakul, N.; Chotigeat, W.; Kedjarune-Leggat, U. Translationally controlled tumor protein against apoptosis from 2-hydroxy-ethyl methacrylate in human dental pulp cells. J. Mater. Sci. Mater. Med. 2011, 22, 1479–1487. [Google Scholar] [CrossRef]
- Amson, R.; Pece, S.; Marine, J.C.; Di Fiore, P.P.; Telerman, A. TPT1/TCTP-regulated pathways in phenotypic reprogramming. Trends Cell Biol. 2012, 23, 37–46. [Google Scholar] [CrossRef]
- Pauli, I.; Minozzo, J.C.; da Silva, P.H.; Chaim, O.M.; Veiga, S.S. Analysis of therapeutic benefits of antivenin at different time intervals after experimental envenomation in rabbits by venom of the brown spider (Loxosceles intermedia). Toxicon 2009, 53, 660–671. [Google Scholar] [CrossRef]
- Felicori, L.; Araujo, S.C.; de Avila, R.A.; Sanchez, E.F.; Granier, C.; Kalapothakis, E.; Chávez-Olórtegui, C. Functional characterization and epitope analysis of a recombinant dermonecrotic protein from Loxosceles intermedia spider. Toxicon 2006, 48, 509–519. [Google Scholar] [CrossRef]
- Duarte, C.G.; Bonilla, C.; Guimarães, G.; Machado de Avila, R.A.; Mendes, T.M.; Silva, W.; Tintaya, B.; Yarleque, A.; Chávez-Olórtegui, C. Anti-loxoscelic horse serum produced against a recombinant dermonecrotic protein of Brazilian Loxosceles intermedia spider neutralize lethal effects of Loxosceles laeta venom from Peru. Toxicon 2014, 93, 37–40. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.M.; Fernandes-Pedrosa, M.F.; de Andrade, R.M.; Marcelino, J.R.; Gondo-Higashi, H.; de Azevedo Ide, L.; Ho, P.L.; van den Berg, C.; Tambourgi, D.V. A new anti-loxoscelic serum produced against recombinant sphingomyelinase D: Results of preclinical trials. Am. J. Trop. Med. Hyg. 2008, 79, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Olvera, A.; Ramos-Cerrillo, B.; Estevez, J.; Clement, H.; de Roodt, A.; Paniagua-Solis, J.; Vazquez, H.; Zavaleta, A.; Arruz, M.S.; Stock, R.P.; et al. North and South American Loxosceles spiders: Development of a polyvalent antivenom with recombinant sphingomyelinases D as antigens. Toxicon 2006, 48, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Dias-Lopes, C.; Guimarães, G.; Felicori, L.; Fernandes, P.; Emery, L.; Kalapothakis, E.; Nguyen, C.; Molina, F.; Granier, C.; Chavez-Olortegui, C. A protective immune response against lethal, dermonecrotic and hemorrhagic effects of Loxosceles intermedia venom elicited by a 27-residue peptide. Toxicon 2009, 55, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.M.; Martins, M.S.; Moura, J.F.; Kalapothakis, E.; Oliveira, J.C.; Mangili, O.C.; Granier, C.; Chávez-Olórtegui, C. Production of monoclonal antibodies capable of neutralizing dermonecrotic activity of Loxosceles intermedia spider venom and their use in a specific immunometric assay. Toxicon 2003, 42, 725–731. [Google Scholar] [CrossRef] [PubMed]
- de Moura, J.; Felicori, L.; Moreau, V.; Guimaraes, G.; Dias-Lopes, C.; Molina, L.; Alvarenga, L.M.; Fernandes, P.; Frézard, F.; Ribeiro, R.R.; et al. Protection against the toxic effects of Loxosceles intermedia spider venom elicited by mimotope peptides. Vaccine 2011, 29, 7992–8001. [Google Scholar] [CrossRef]
- Mendes, T.M.; Oliveira, D.; Figueiredo, L.F.; Machado-de-Avila, R.A.; Duarte, C.G.; Dias-Lopes, C.; Guimarães, G.; Felicori, L.; Minozzo, J.C.; Chávez-Olortegui, C. Generation and characterization of a recombinant chimeric protein (rCpLi) consisting of B-cell epitopes of a dermonecrotic protein from Loxosceles intermedia spider venom. Vaccine 2013, 31, 2749–2755. [Google Scholar] [CrossRef]
- Figueiredo, L.F.; Dias-Lopes, C.; Alvarenga, L.M.; Mendes, T.M.; Machado-de-Avila, R.A.; McCormack, J.; Minozzo, J.C.; Kalapothakis, E.; Chávez-Olórtegui, C. Innovative immunization protocols using chimeric recombinant protein for the production of polyspecific loxoscelic antivenom in horses. Toxicon 2014, 86, 59–67. [Google Scholar] [CrossRef]
- Almeida-Lima, S.; Guerra-Duarte, C.; Costal-Oliveira, F.; Mendes, T.M.; Figueiredo, L.F.M.; Oliveira, D.; Ávila, R.A.M.; Ferrer, V.P.; Trevisan-Silva, D.; Veiga, S.S.; et al. Recombinant protein containing B-cell epitopes of different Loxosceles spider toxins generates neutralizing antibodies in immunized rabbits. Front. Immunol. 2018, 9, 653. [Google Scholar] [CrossRef]

| Toxin Family | MM (kDa) | Species | Biological Characteristics | N° of Sequences | PDB |
|---|---|---|---|---|---|
| PLD | 30–35 | L. arizonica [45] L. boneti [36] L. gaucho [46] L. intermedia [28] L. laeta [32] L. reclusa [34] L. similis [35] | -Hydrolysis of phospholipids; -Transphosphatidylation; -Dermonecrosis; -Inflammatory response; -Lethality; -Hemolysis; -Platelet aggregation; -Edema; -Nephrotoxicity; -Cytotoxicity; -Cytokine activation; -Complement activation. | 199 | 1XX1 2F9R 3RLH 3RLG 4RW5 4RW3 |
| Metalloprotease | 30 | L. intermedia [42] | -Hydrolysis of Gelatin, Fibronectin and Fibrinogen; -Cytotoxicity. | 3 | N.A. |
| ICK peptides | 12 | L. intermedia [44] | -Insecticidal activity. | 1 | N.A. |
| Hyaluronidase | 45 | L. intermedia [6] | -Hydrolysis of hyaluronic acid and chondroitin sulfate; -Dermonecrosis spreading. | 1 | N.A. |
| TCTP | 22 | L. intermedia [10] | -Edema; -Vascular permeability. | 1 | N.A. |
| Toxin Family | Potential Uses as Biotools | Potential Uses for Drugs Design |
|---|---|---|
| Phospholipase-D [22,23,24,27,33,69,105] | -Antigens for a specific serum production for serum therapy; -Antigens for putative laboratory diagnosis tests; -Production of lipids for industrial interest; -Emulsification-free degumming of oil; | -Treatment of Loxoscelism; -Anti-inflammatory drugs, -Neuroprotective drugs; -Adjuvant drugs for cancer chemotherapy; |
| Metalloprotease [41,42,43] | -Trombolytic agents | Treatment of atherosclerosis |
| ICK peptides [7,44,64] | -Use as Bioinsecticide -Neuroprotective effect | Analgesic drugs |
| Hyaluronidase [6,9,78] | -Adjuvant for drugs absorption -Resorption of fluids -Diagnostic allergy test -Delivery of chemotherapy | Contraceptive method |
| Serpin [9,78] | -Inflammatory modulation -Agricultural pest regulators | Antitumoral drugs |
| TCTP [9,10,78] | -Antiparasitic effect -Dental restoration -Drug delivery | -N.A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves-Moreira, D.; Matsubara, F.H.; Schemczssen-Graeff, Z.; De Bona, E.; Heidemann, V.R.; Guerra-Duarte, C.; Gremski, L.H.; Chávez-Olórtegui, C.; Senff-Ribeiro, A.; Chaim, O.M.; et al. Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics. Toxins 2019, 11, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060355
Chaves-Moreira D, Matsubara FH, Schemczssen-Graeff Z, De Bona E, Heidemann VR, Guerra-Duarte C, Gremski LH, Chávez-Olórtegui C, Senff-Ribeiro A, Chaim OM, et al. Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics. Toxins. 2019; 11(6):355. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060355
Chicago/Turabian StyleChaves-Moreira, Daniele, Fernando Hitomi Matsubara, Zelinda Schemczssen-Graeff, Elidiana De Bona, Vanessa Ribeiro Heidemann, Clara Guerra-Duarte, Luiza Helena Gremski, Carlos Chávez-Olórtegui, Andrea Senff-Ribeiro, Olga Meiri Chaim, and et al. 2019. "Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics" Toxins 11, no. 6: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060355





