An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation
Abstract
:1. Introduction
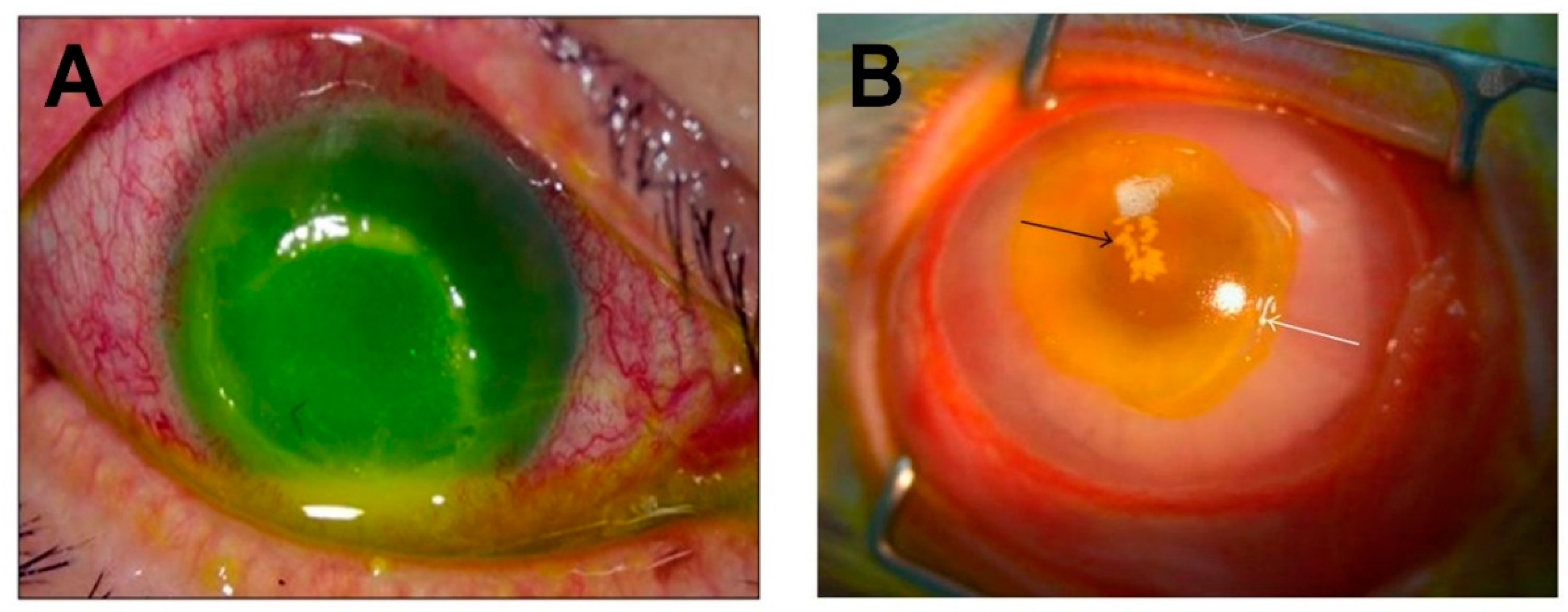
2. S. aureus and Eye Infections
3. Membrane-Damaging Toxins
4. Bi-Component Toxins
5. Enzymes
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ogston, A. Report upon micro-organisms in surgical diseases. Br. Med. J. 1881, 1, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, A. Staphylococcus Aureus; Academic Press: Cambridge, MA, USA, 2018; p. 3. [Google Scholar]
- Rosenbach, F.J. Mikro-organismen bei den Wund-Infection-krankheiten des Menschen; Wiesbaden, J.F. Bergmann: Plano, TX, USA, 1884; pp. 19–21. [Google Scholar]
- Hooker, C. Diphtheria, Immunization and the Bundaberg Tragedy: A Study of Public Health in Australia. Health Hist. 2000, 2, 52–78. [Google Scholar] [CrossRef]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Burnet, F.M. The Exotoxins of Staphylococcus Pyogenes Aureus. J. Pathol. Bacteriol. 1929, 32, 717–734. [Google Scholar] [CrossRef]
- Burnet, F.M. The Production of Staphylococcal Toxin. J. Pathol. Bacteriol. 1930, 33, 1–16. [Google Scholar] [CrossRef]
- Burky, E.L. Studies on Cultures and Broth Filtrates of Staphylococci. J. Immunol. 1933, 25, 419–437. [Google Scholar]
- Woolpert, O.C.; Dack, G.M. Relation of Gastro-Intestinal Poison to Other Toxic Substances Produced by Staphylococci. J. Infect. Dis. 1933, 52, 6–19. [Google Scholar] [CrossRef]
- Bigger, J.W. The Production of Staphylococcal Haemolysin With Observations on Its Mode of Action. J. Pathol. Bacteriol. 1933, 36, 87–114. [Google Scholar] [CrossRef]
- Glenny, A.T.; Stevens, M.F. Staphylococcus Toxins and Antitoxins. J. Pathol. Bacteriol. 1935, 40, 201–210. [Google Scholar] [CrossRef]
- Kumar, S.; Lindorfer, R.K. The Characterization of Staphylococcal Toxins. J. Exp. Med. 1962, 30, 1095–1106. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Schwartz, L.L. Isolation and Composition of Staphylococcal Alpha Toxin. J. Gen. Microbiol. 1963, 30, 455–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowy, F.D. Staphylococcus Aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Sollid, J.U.E.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinates of human carriage. Infect. Genet. Evolut. 2014, 21, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Vos, M.C.; Ott, A.; van Belkum, A.; Voss, A.; Kluytmans, J.; van Keulen, P.; Vandenbroucke-Grauls, C.; Meester, M.; Verbrugh, H. Risk and outcome of nosocomial Staphylococcus aureus bacteremia in nasal carriers versus non-carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Lebon, A.; Moll, H.A.; Tavakol, M.; van Wamel, W.J.; Jaddoe, V.; Hofman, A.; Verbrugh, H.A.; van Belkum, A. Correlation of Bacterial Colonization Status between Mother and Child: The Generation R. Study. J. Clin. Microbiol. 2010, 48, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Gorwitz, R.J.; Kruszon-Moran, D.; McAllister, S.K.; McQuillan, G.; McDougal, L.K.; Fosheim, G.E.; Jensen, B.J.; Killgore, G.; Tenover, F.C.; Kuehnert, M.J. Changes in the Prevalence of Nasal Colonization with Staphylococcus aureus in the United States 2001–2004. J. Infect. Dis. 2008, 197, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Uemura, E.; Kakinohana, S.; Higa, N.; Toma, C.; Nakasone, N. Comparative characterization of Staphylococcus aureus isolates from throats and noses of healthy volunteers. Jpn. J. Infect. Dis. 2004, 57, 21–24. [Google Scholar]
- Saxena, S.; Singh, K.; Talwar, V. Methicillin-resistant Staphylococcus aureus prevalence in community in the East Delhi area. Jpn. J. Infect. Dis. 2003, 56, 54–56. [Google Scholar]
- Omuse, G.; Kariuki, S.; Revathi, G. Unexpected absence of methicillin-resistant Staphylococcus aureus nasal carriage by healthcare workers in a tertiary hospital in Kenya. J. Hosp. Infect. 2012, 80, 71–73. [Google Scholar] [CrossRef]
- Hamdan-Partida, A.; Sainz-Espunes, T.; Bustos-Martinez, J. Characterization and Persistence of Staphylococcus aureus Strains Isolated from the Anterior Nares and Throats of Healthy Carriers in a Mexican Community. J. Clin. Microbiol. 2010, 48, 1701–1705. [Google Scholar] [CrossRef]
- Eriksen, N.; Espersen, F.; Rosdahl, V.T.; Jensen, K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 1995, 115, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; van Belkum, A.; Verbruch, H. Nasal Carriage of Staphylococcus aureus: Epidemiology, Underlying Mechanisms, and Associated Risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Acton, D.S.; Plat-Sinnige, M.J.; van Wamel, W.; de Groot, N.; van Belkum, A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its impact? Eur. Clin. Microbiol. Infect. Dis. 2009, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of Resistance: Staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R. The Discovery of Penicillin–New Insights After More Than 75 years of Clinical Use. Emerging Infect. Dis. J. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Fleming, A. Penicillin its Practical Applications, 2nd ed.; Butterworth & Co.: London, UK, 1950; p. 15. [Google Scholar]
- Kirby, W.M.M. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science 1944, 99, 452–453. [Google Scholar] [CrossRef]
- Barber, M.; Rozwadowska-Dowzenko, M. Infection by Penicillin-Resistant Staphylococci. Lancet 1948, 23, 641–644. [Google Scholar] [CrossRef]
- Blair, J.E.; Carr, M. Distribution of Phage Groups of Staphylococcus aureus in the Years 1927 through 1947. Science 1960, 132, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Jevons, M.P.; Parker, M.T. The evolution of new hospital strains of Staphylococcus aureus. J. Clin. Pathol. 1964, 17, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Chambers, H.F. Methicillin-Resistant Staphylococci. Clin. Microbiol. Rev. 1988, 1, 173–186. [Google Scholar] [CrossRef]
- Barber, M. Methicillin-resistant staphylococci. J. Clin. Pathol. 1961, 14, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. 2018, 4, 1188–1196. [Google Scholar]
- Vandensch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Liassine, N.; Bes, M.; Greenland, T.; Reverdy, M.; et al. Community-Acquired Methicillin-Resistant Staphylococcus aureus Carrying Panton-Valentine Leukocidin Genes: Worldwide Emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, J.R.; Chen, L.; Mathema, B.; Kreiswirth, B.N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 2012, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Boswihi, S.S.; Udo, E.E. Methicillin-resistant Staphylococcus aureus: An update on the epidemiology, treatment options and infection control. Curr. Med. Res. Pract. 2018, 8, 18–24. [Google Scholar] [CrossRef]
- Blomquist, P.H. Methicillin-Resistant Staphylococcus aureus Infections of the Eye and Orbit. Trans. Am. Ophthalmol. Soc. 2006, 104, 322–345. [Google Scholar]
- Hsiao, C.; Chuang, C.; Tan, H.; Ma, D.; Lin, K.; Chang, C.; Huang, Y. Methicillin-Resistant Staphylococcus aureus Ocular Infection: A 10-Year Hospital-Based Study. Ophthalmology 2012, 119, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.; Hsiao, C.; Tan, H.; Ma, D.; Lin, K.; Chang, C.; Huang, Y. Staphylococcus aureus Ocular Infection: Methicillin-Resistance, Clinical Features, and Antibiotic Susceptibilities. PLoS ONE 2012, 8, e42437. [Google Scholar] [CrossRef]
- Brian, G.; Taylor, H. Cataract blindness-challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar]
- Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J. Cataract Refract. Surg. 2007, 33, 978–988. [Google Scholar] [CrossRef]
- Taban, M.; Behrens, A.; Newcomb, R.L.; Nobe, M.Y.; Saedi, G.; Sweet, P.M.; McDonnell, P.J. Acute endophthalmitis following cataract surgery: A systemic review of the literature. Arch. Ophthalmol. 2005, 123, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Lad, E.M.; Moshfeghi, D.M. Intravitreal injection of therapeutic agents. Retina 2009, 29, 875–912. [Google Scholar] [CrossRef] [PubMed]
- Rupenthal, I.D. Sector Overview Ocular Drug Delivery: Exciting Times Ahead. Available online: http://ondrugdelivery.com/publications/54/Sector_Overview.pdf (accessed on 1 June 2019).
- Sadaka, A.; Durand, M.L.; Gilmore, M.S. Bacterial endophthalmitis in the age of outpatient intravitreal therapies and cataract surgeries: Host-microbe interactions in intraocular infection. Prog. Retin. Eye Res. 2012, 31, 316–331. [Google Scholar] [CrossRef] [PubMed]
- VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group; D’Amico, D.J.; Masonson, H.N.; Patel, M.; Adamis, A.P.; Cunningham, E.T., Jr.; Guyer, D.R.; Katz, B. Pegaptanib sodium for neovascular age-related macular degeneration: Two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006, 113, 992–1001.e6. [Google Scholar]
- Diago, T.; McCannel, C.A.; Bakri, S.J.; Pulido, J.S.; Edwards, A.O.; Pach, J.M. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina 2009, 29, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.S.; Walsh, M.K.; Hassan, T.S.; Halperin, L.S.; Castellarian, A.A.; Roth, D.; Driscoll, S.; Prenner, J.L. Endophthalmitis after anti-VEGF injections. Ophthalmology 2009, 116, 1225. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Engelbert, M.; Parke, D.W.; Jett, B.D.; Gilmore, M.S. Bacterial Endophthalmitis: Epidemiology, Therapeutics, and Bacterium-Host Interactions. Clin. Microbiol. Rev. 2002, 15, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Grumann, D.; Nubel, U.; Borker, B.M. Staphylococcus aureus toxins–Their functions and genetics. Infect. Genet. Evolut. 2014, 21, 583–592. [Google Scholar] [CrossRef]
- Marquart, M.E.; O’Callaghan, R.J. Infectious Keratitis: Secreted Bacterial Proteins That Mediate Corneal Damage. J. Ophthalmol. 2013, 2013, 369094. [Google Scholar] [CrossRef]
- O’Callaghan, R.J. The Pathogenesis of Staphylococcus aureus Eye Infections. Pathogens 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, F.; Torres, V.J. Bacterial Survival Amidst and Immune Onslaught: The Contribution of the Staphylococcus aureus Leukotoxins. PLoS Pathog. 2013, 9, e1003143. [Google Scholar] [CrossRef] [PubMed]
- Inoshima, I.; Inoshima, N.; Wilke, G.A.; Powers, M.E.; Frank, K.M.; Wang, Y.; Wardenburg, J.B. Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 2011, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Perret, M.; Badiou, C.; Lina, G.; Burbaud, S.; Benito, Y.; Bes, M.; Cottin, V.; Couzon, F.; Juruj, C.; Dauwalder, O.; et al. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells trigger chemokine secretion in an inflammasome-dependent manner. Cell. Microbiol. 2012, 14, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- Seilie, E.S.; Wardenburg, J.B. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 2017, 72, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; van der Goot, F. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Kinoshita, S. Innate immunity of the ocular surface. Brain Res. Bull. 2010, 81, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Kinoshita, S. Ocular surface inflammation is regulated by innate immunity. Prog. Retin. Eye Res. 2012, 31, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Foulsham, W.; Coco, G.; Amouzegar, A.; Chauhan, S.K.; Dana, R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends Immunol. 2018, 39, 288–301. [Google Scholar] [CrossRef]
- Booth, M.C.; Pence, L.M.; Mahasreshti, P.; Callegan, M.C.; Gilmore, M.S. Clonal Associations among Staphylococcus aureus Isolates from Various Sites of Infection. Infect. Immun. 2001, 69, 345–352. [Google Scholar] [CrossRef]
- Kielian, T.; Cheung, A.; Hickey, W.F. Diminished Virulence of an Alpha-Toxin Mutant of Staphylococcus aureus in Experimental Brain Abscesses. Infect. Immun. 2001, 69, 6902–6911. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Ramos, M.D.; Menzies, B.E.; Yeaman, M.R.; Shen, A.J.; Cheung, A.L. Hyperproduction of Alpha-Toxin by Staphylococcus aureus Results in Paradoxically Reduced Virulence in Experimental Endocarditis: A Host Defense Role for Platelet Microbicidal Proteins. Infect. Immun. 1997, 65, 4652–4660. [Google Scholar] [PubMed]
- Callegan, M.C.; Engel, L.S.; Hill, J.M.; O’Callaghan, R.J. Corneal Virulence of Staphylococcus aureus: Roles of Alpha-Toxin and Protein A in Pathogenesis. Infect. Immun. 1994, 62, 2478–2482. [Google Scholar] [PubMed]
- O’Callaghan, R.J.; Callegan, M.C.; Moreau, J.M.; Green, L.C.; Foster, T.J.; Hartford, O.M.; Engel, L.S.; Hill, J.M. Specific Roles of Alpha-toxin and Beta-Toxin during Staphylococcus aureus Corneal Infection. Infect. Immun. 1997, 65, 1571–1578. [Google Scholar] [PubMed]
- Jonsson, P.; Lindberg, M.; Haraldsson, I.; Wadstrom, T. Virulence of Staphylococcus aureus in a Mouse Mastitis Model: Studies of Alpha Hemolysin, Coagulase, and Protein A as Possible Virulence Determinates with Protoplast Fusion and Gene Cloning. Infect. Immun. 1985, 49, 765–769. [Google Scholar] [PubMed]
- Wardenburg, J.B.; Patel, R.J.; Schneewind, O. Surface Proteins and Exotoxins Are Required for the Pathogenesis of Staphylococcus aureus Pneumonia. Infect. Immun. 2007, 75, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Wardenburg, J.B.; Bae, T.; Otto, M.; Deleo, F.R.; Schneewind, O. Poring over pores: Alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 2007, 13, 1405–1406. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.E.; Kernodle, D.S. Passive Immunization with Antiserum to a Nontoxic Alpha-Toxin Mutant from Staphylococcus aureus Is Protective in a Murine Model. Infect. Immun. 1996, 64, 1839–1841. [Google Scholar] [PubMed]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Wardenburg, J.B. ADAM10 Mediates Vascular Injury Induced by Staphylococcus aureus α-Hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Wardenburg, J.B.; Gardner, D.J.; Long, D.; Whitney, A.R.; Braughton, K.R.; Schneewind, O.; DeLeo, F.R. Targeting of Alpha-Hemolysin by Active or Passive Immunization Decreases Severity of USA300 Skin Infection in a Mouse Model. J. Infect. Dis. 2010, 202, 1050–1058. [Google Scholar] [CrossRef]
- Patel, A.H.; Nowland, P.; Weavers, E.D.; Foster, T. Virulence of Protein A-Deficient and Alpha-Toxin-Deficient Mutants of Staphylococcus aureus Isolated by Allele Replacement. Infect. Immun. 1987, 55, 3103–3110. [Google Scholar] [PubMed]
- Wilke, G.A.; Wardenburg, J.B. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [PubMed]
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Austin, M.S.; Sloop, G.D.; Moreau, J.M.; Hume, E.B.H.; Thompson, H.W.; McAleese, F.M.; Foster, T.J.; O’Callaghan, R.J. Corneal Pathogenesis of Staphylococcus aureus Strain Newman. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1109–1115. [Google Scholar]
- Girgis, D.O.; Sloop, G.D.; Reed, J.M.; O’Callaghan, R.J. Effects of Toxin Production in a Murine Model of Staphylococcus aureus Keratitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Putra, I.; Rabiee, B.; Anwar, K.N.; Gidfar, S.; Shen, X.; Babalooee, M.; Ghassemi, M.; Afsharkhamseh, N.; Bakhsh, S.; Missiakas, D.; et al. Staphylococcus aureus Alpha-Hemolysin Impairs Corneal Epithelial Wound Healing and Promotes Intracellular Bacterial Invasion. Exp. Eye Res. 2019, 181, 263–270. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Kim, M. Two Cases of Corneal Ulcer due to Methicillin-Resistant Staphylococcus aureus in High Risk Groups. Korean J. Ophthalmol. 2010, 24, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E. Animal Models of Bacterial Keratitis. J. Biomed. Biotechnol. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, R.J.; McCormick, C.C.; Caballero, A.R.; Marquart, M.E.; Gatlin, H.P.; Fratkin, J.D. Age-Related Differences in Rabbits during Experimental Staphylococcus aureus Keratitis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5125–5131. [Google Scholar] [CrossRef]
- Moreau, J.M.; Sloop, G.D.; Engel, L.S.; Hill, J.M.; O’Callaghan, J. Histopathological studies of staphylococcal alpha-toxin: Effects on rabbit corneas. Curr. Eye Res. 1997, 16, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. Role of Staphylococcus aureus Virulence Factors in Inducing Inflammation and Vascular Permeability in a Mouse Model of Bacterial Endophthalmitis. PLoS ONE 2015, 10, e0128423. [Google Scholar] [CrossRef]
- Goerke, C.; Fluckiger, U.; Steinhuber, A.; Zimmerli, W.; Wolz, C. Impact of the regulatory loci agr, sarA, and sae of Staphylococcus aureus on the induction of α-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microb. 2001, 40, 1439–1447. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Willard, J.; Yeaman, M.R.; Cheung, A.L.; Bayer, A.S. Regulation of Staphylococus aureus α-Toxin Gene (hla) Expression by agr, sarA, and sae In Vitro and in Experimental Infective Endocarditis. J. Infect. Dis. 2006, 194, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.C.; Cheung, A.L.; Hatter, K.L.; Jett, B.D.; Callegan, M.C.; Gilmore, M.S. Staphylococcal Accessory Regulator (sar) in Conjunction with agr Contributes to Staphylococcus aureus Virulence in Endophthalmitis. Infect. Immun. 1997, 65, 1550–1556. [Google Scholar] [PubMed]
- Horsburgh, M.J.; Aish, J.L.; White, I.J.; Shaw, L.; Lithgow, J.K.; Foster, S.J. σB Modulates Virulence Determinant Expression and Stress Resistance: Characterization of a Functional rsbU Strain Derived from Staphylococcus aureus 8325-4. J. Bacteriol. 2002, 184, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Coburn, P.S.; Miller, F.C.; LaGrow, A.L.; Land, C.; Mursalin, H.; Livingston, E.; Amayem, O.; Chen, Y.; Gao, L.; Callegan, M.C. Disarming Pore-Forming Toxins with Biomimetic Nanosponges in Intraocular Infections. mSphere 2019, 4, e00262-19. [Google Scholar] [CrossRef] [Green Version]
- Gravet, A.; Colin, D.A.; Keller, R.; Giradot, R.; Monteil, H.; Prevost, G. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 1998, 136, 202–208. [Google Scholar] [CrossRef]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.C.; van Hooijdonk, D.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential Interactions of the Staphylococcal Toxins Panton-Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef]
- Spaan, A.N.; van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. 2017, 15, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.; van Kessel, K.; Vandenesch, F.; et al. The Staphylocccal Toxin Panton-Valentine Leukocidin Targets Human C5a Receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, T.; Zaidi, T.; Yoong, P.; Pier, G.B. Staphylococcus aureus Corneal Infections: Effect of the Panton-Valentine Leukocidin (PVL) and Antibody to PVL on Virulence and Pathology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.A.; Speeg-Schatz, C.; Freitas, F.I.S.; Sahel, J.; Monteil, H.; Prevost, G. Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model. J. Med. Microbiol. 1997, 46, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Heitz, P.; Roux, M.; Keller, D.; Bourcier, T.; Sauer, A.; Pervost, G.; Gaucher, D. Panton-Valentine Leukocidin Colocalizes with Retinal Ganglion and Amacrine Cells and Acivates Glial Reactions and Microglial Apoptosis. Sci. Rep. 2018, 8, 2953. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Roux, M.J.; Picaud, S.; Keller, D.; Sauer, A.; Heitz, P.; Pervost, G.; Gaucher, D. Panton-Valentine Leucocidin Proves Direct Neuronal Targeting and Its Early Neuronal and Glial Impacts a Rabbit Retinal Explant Model. Toxins 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C.; Durkee, H.; Miller, D.; Maestre-Mesa, J.; Arboleda, A.; Aguilar, M.C.; Relhan, N.; Flynn, H.W.; Amescua, G.; Parel, J.; et al. Molecular epidemiology and resistance profiles among healthcare- and community-associated Staphylococcus aureus keratitis isolates. Infect. Drug Resist. 2019, 12, 831–843. [Google Scholar] [CrossRef] [PubMed]
- von Eiff, C.; Friedrich, A.W.; Peters, G.; Becker, K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2004, 49, 157–162. [Google Scholar] [CrossRef]
- Malachowa, N.; Whitney, A.R.; Kobayashi, S.D.; Sturdevant, D.E.; Kennedy, A.D.; Braughton, K.R.; Shabb, D.W.; Diep, B.A.; Chambers, H.F.; Otto, M.; et al. Global Changes in Staphylococcus aureus Gene Expression in Human Blood. PLoS ONE 2011, 6, e18617. [Google Scholar] [CrossRef]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Said-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardener, D.J.; Kreiswirth, B.N.; et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; de Haas, C.; Day, C.J.; Jennings, M.P.; et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef] [PubMed]
- Supersac, G.; Piemont, Y.; Kubina, M.; Prevost, G.; Foster, T.J. Assessment of the role of γ-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb. Pathog. 1998, 24, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Dassy, B.; Hogan, T.; Foster, T.J.; Fournier, J. Involvement of the accessory gene regulator (agr) in expression of the type 5 capsular polysaccharide by Staphylococcus aureus. Microbiology 1993, 139, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Bae, T.; Schneewind, O.; Takeuchi, F.; Hiramatsu, K. Genome Sequence of Staphylococcus aureus Strain Newman and Comparative Analysis of Staphylococcal Genomes: Polymorphism and Evolution of Two Major Pathogenicity Islands. J. Bacteriol. 2008, 190, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. Phenol-soluble modulins in staphylococci What are they originally for? Commun. Integr. Biol. 2012, 5, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Kelbanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinates for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Baek, K.T.; Frees, D.; Renzoni, A.; Barras, C.; Rodriguez, N.; Manzano, N.; Kelley, W.L. Genetic Variation in the Staphylococcus aureus 8325 Strain Lineage Revealed by Whole-Genome Sequencing. PLoS ONE 2013, 8, e77122. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef]
- Hanzelmann, D.; Joo, H.; Franz-Wachel, M.; Hertlein, T.; Stevanovic, S.; Macek, B.; Wolz, C.; Gotz, F.; Otto, M.; Krestschmer, D.; et al. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 2016, 7, 12304. [Google Scholar] [CrossRef]
- Kretschmer, D.; Gleske, A.; Rautenberg, M.; Wang, R.; Koberle, M.; Bohn, E.; Schoneberg, T.; Rabiet, M.; Boulay, F.; Klebanoff, S.J.; et al. Human Formyl Peptide Receptor 2 Senses Highly Pathogenic Staphylococcus aureus. Cell Host Microbe 2010, 7, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.; Hanzelmann, D.; Fehlhaber, B.; Klos, A.; von Leowenich, F.; Liese, J.; Peschel, A.; Kretschmer, D. Formyl-peptide receptor 2 governs leukocyte influx in local Staphylococcus aureus infections. FASEB J. 2018, 32, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Freer, J.H.; Arbuthnott, J.P. Toxins of Staphylococcus aureus. Pharmacol. Ther. 1983, 19, 55–106. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Larsen, H.D.; Eriksen, N.H.R.; Elsberg, C.S.; Jensen, N.E. Frequency of α-and β-haemolysin in Staphylococcus aureus of bovine and human origin. Apmis 1999, 107, 425–430. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, W.; Rooijakkers, S.; Ruyken, M.; van Kessel, K.; van Strijp, J. The Innate Immune Modulators Staphylococcal Complement Inhibitor and Chemotaxis Inhibitory Protein of Staphylococcus aureus Are Located on β-Hemolysin-Converting Bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Pabon, W.; Herrera, A.; Vu, B.G.; Stach, C.S.; Merriman, J.A.; Spaulding, A.R.; Schlievert, P.M. Staphylococcus aureus β-toxin Production is Common in Strains with the β-toxin Gene Inactivation by Bacteriophage. J. Infect. Dis. 2014, 210, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Walev, I.; Weller, U.; Strauch, S.; Foster, T.; Bhakdi, S. Selective Killing of Human Monocytes and Cytokine Release Provoked by Sphingomyelinase (Beta-Toxin) of Staphylococcus aureus. Infect. Immun. 1996, 64, 2974–2979. [Google Scholar]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [Green Version]
- Warren, H.S.; Tompkins, R.G.; Modawer, L.L.; Seok, J.; Xu, W.; Mindrinos, M.N.; Maier, R.V.; Xiao, W.; Davis, R.W. Mice are not men. Proc. Natl. Acad. Sci. USA 2015, 112, E345. [Google Scholar] [CrossRef]
- Astley, R.A.; Coburn, P.S.; Parkunan, S.M.; Callegan, M.C. Modeling intraocular bacterial infections. Prog. Retina Eye Res. 2016, 54, 30–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, B.; Hazlett, L.D. Association of CD4+ T Cell-Dependent Keratitis with Genetic Susceptibility to Pseudomonas aeruginosa Ocular Infection. J. Immunol. 1997, 159, 6283–6290. [Google Scholar] [PubMed]


| Gene | MSSA (7) | MRSA (9) | All (16) |
|---|---|---|---|
| hla | 6 (86%) | 9 (100%) | 15 (94%) |
| hlb | 4 (57%) | 5 (56%) | 8 (50%) |
| mecA | 0 | 9 | 9 |
| Toxin Absent | S. Aureus Strain | Ocular Infection Model | Result | References |
|---|---|---|---|---|
| α-toxin | 8325-4 | Keratitis rabbit | 🡫 Slit lamp scores 🡫 Myeloperoxidase activity 🡫 Corneal erosions | [67] |
| α-toxin | 8325-4 | Keratitis rabbit | 🡫 Slit lamp scores 🡫 Inflammation | [68] |
| β-toxin | 8325-4 | Keratitis rabbit | 🡫 Scleral edema | [68] |
| γ-toxin | Newman | Endophthalmitis rabbit | 🡫 Lid inflammation | [106] |
| α-toxin | Newman | Keratitis rabbit | 🡫 Slit lamp scores 🡫 Inflammation | [81] |
| γ-toxin | Newman | Keratitis rabbit | 🡫 Slit lamp scores 🡫 Corneal PMN | [81] |
| α-toxin | 8325-4 | Endophthalmitis rabbit | 🡫 Retinal damage | [52] |
| β-toxin | 8325-4 | Endophthalmitis rabbit | 🡫 Retinal damage | [52] |
| γ-toxin | 8325-4 | Endophthalmitis rabbit | No change | [52] |
| α-toxin | 8325-4 | Keratitis mouse | More severe in aged mice | [80] |
| PVL | Various USA 300 and 400 strains | Keratitis mouse | Enhanced virulence in a subset of MRSA strains | [97] |
| α-toxin | JE2 | Keratitis mouse | 🡡 Corneal healing | [81] |
| Toxin | Inoculation Method | Ocular Model | Result | References |
|---|---|---|---|---|
| α-toxin | Topical | Rabbit cornea | Inflammation of conjunctiva and iris | [68] |
| α-toxin | Injection | Rabbit cornea | Inflammation of cornea and iris, corneal epithelial defect | [68] |
| β-toxin | Topical | Rabbit cornea | Inflammation of conjunctiva | [68] |
| β-toxin | Injection | Rabbit cornea | Scleral edema | [68] |
| γ-toxin | Injection | Rabbit cornea | Acute inflammatory reactions | [98] |
| PVL | Injection | Rabbit cornea | Acute inflammatory reactions | [98] |
| α-toxin | Injection | Rabbit cornea | ↑SLE score, edema, epithelia cell death | [85] |
| α-toxin | Topical | Mouse cornea | Corneal pathology more severe in aged mice | [80] |
| α-toxin | Injection | Rabbit cornea | Corneal pathology more severe in young rabbits | [81] |
| α-toxin | Injection | Mouse Vitreous | Mild retinal damage, no reduction in retinal function | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astley, R.; Miller, F.C.; Mursalin, M.H.; Coburn, P.S.; Callegan, M.C. An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation. Toxins 2019, 11, 356. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060356
Astley R, Miller FC, Mursalin MH, Coburn PS, Callegan MC. An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation. Toxins. 2019; 11(6):356. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060356
Chicago/Turabian StyleAstley, Roger, Frederick C. Miller, Md Huzzatul Mursalin, Phillip S. Coburn, and Michelle C. Callegan. 2019. "An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation" Toxins 11, no. 6: 356. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11060356






