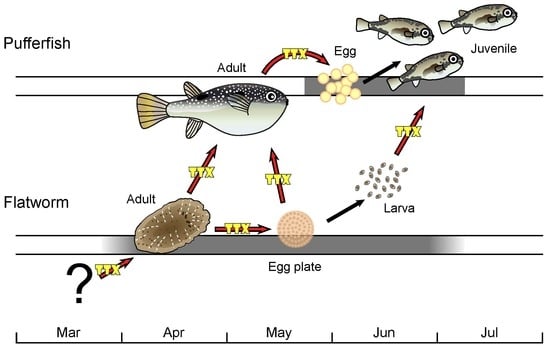
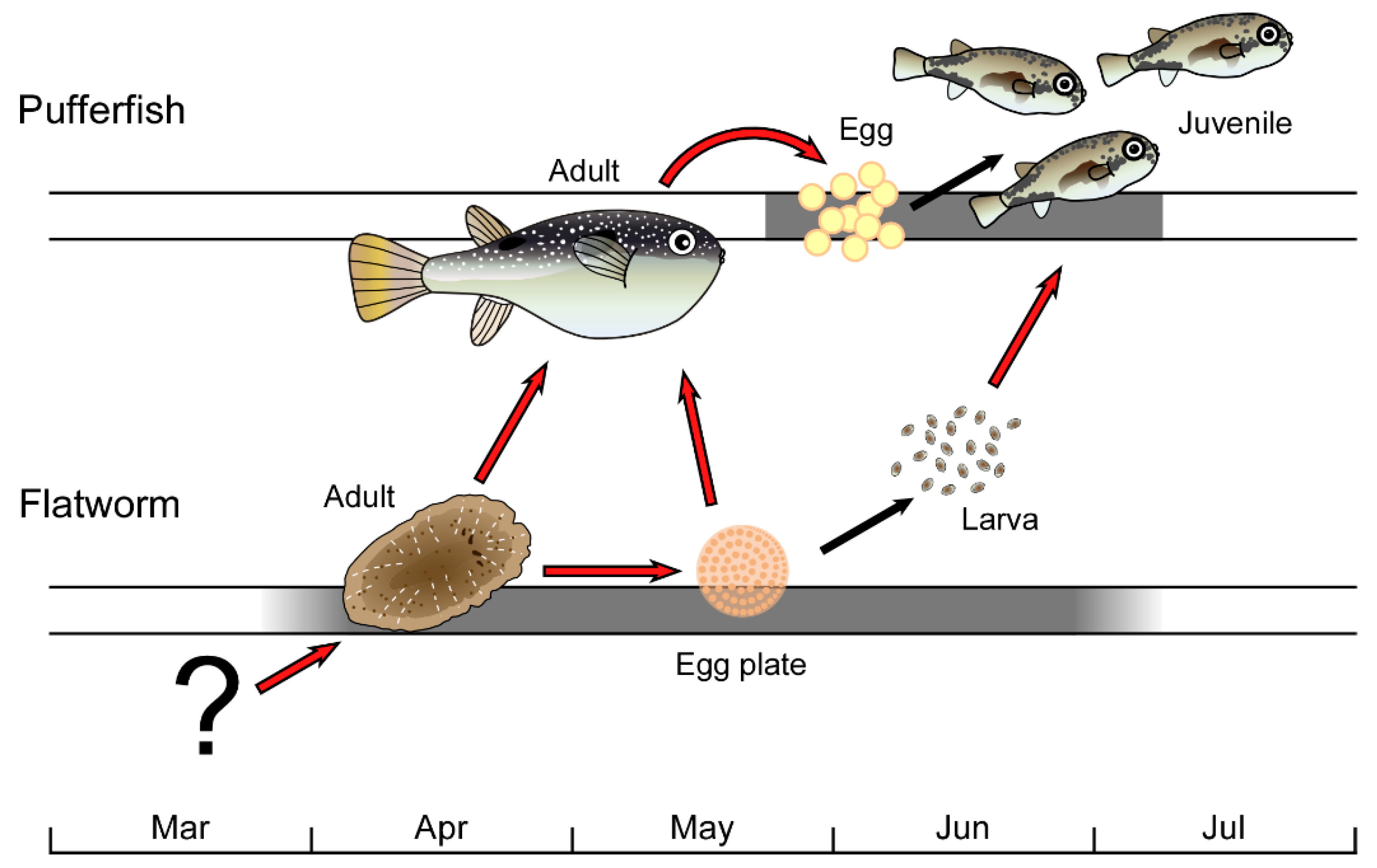
Toxic Flatworm Egg Plates Serve as a Possible Source of Tetrodotoxin for Pufferfish
Abstract
:1. Introduction
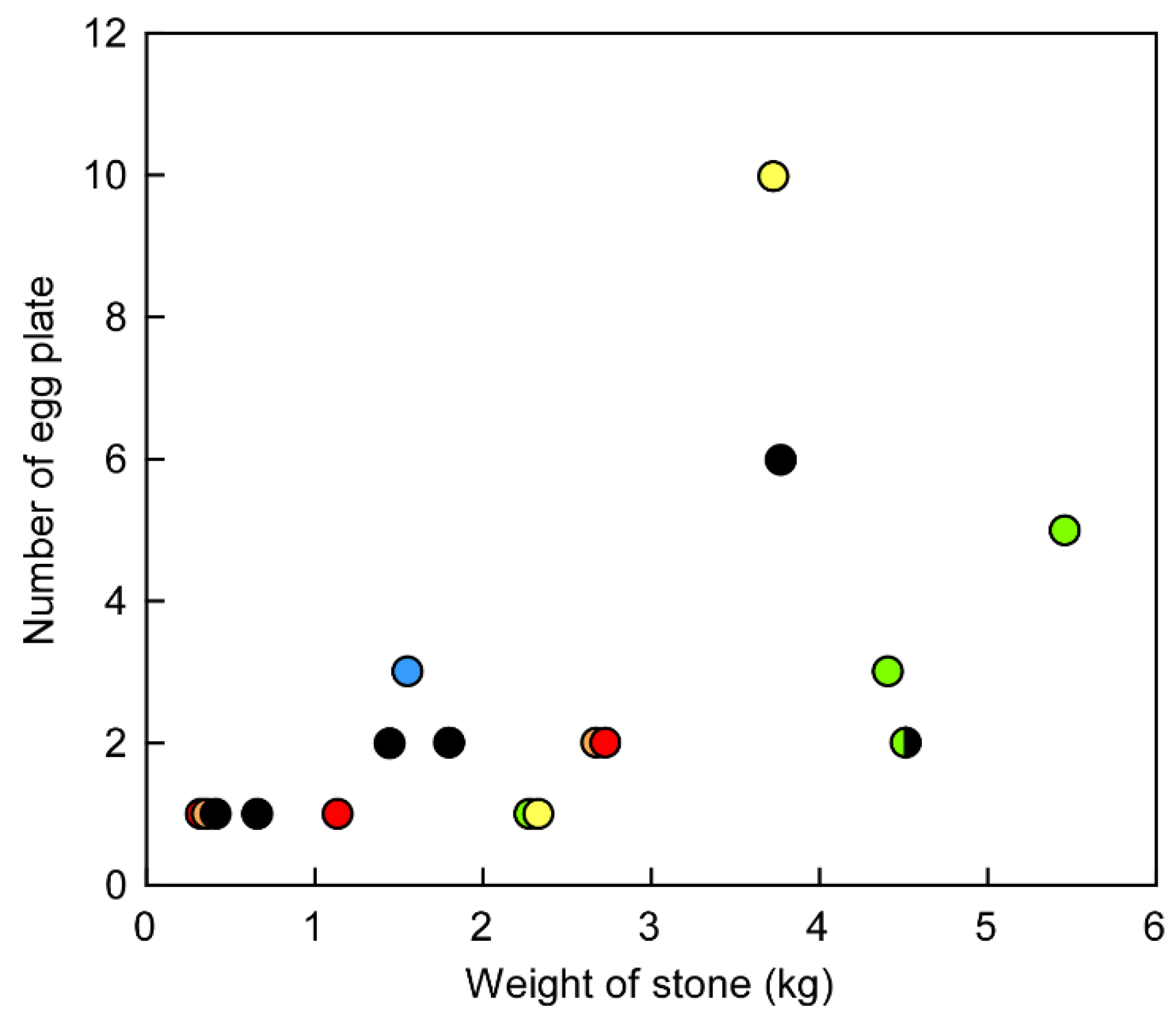
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Observation of Feeding Behavior in the Natural Environment
4.2. Feeding Experiment in the Laboratory
4.3. PCR Amplification and DNA Sequencing
4.4. LC-MS/MS Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Colquhon, D.; Henderson, R.; Ritchie, J.M. The binding of labeled tetrodotoxin to non-myelineated nerve fibres. J. Physiol. 1972, 227, 95–126. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Pharmacology of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 67–84. [Google Scholar] [CrossRef]
- Kim, Y.H.; Brown, G.B.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 1975, 189, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Mosher, H.S.; Fuhrman, G.J.; Fuhrman, F.A.; Fischer, H.G. Tarichatoxin-tetrodotoxin, a potent neurotoxin. Science 1964, 144, 1100. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. D 2006, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Hashimoto, K. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon 1973, 11, 305–307. [Google Scholar] [CrossRef]
- Noguchi, T.; Uzu, A.; Koyama, K.; Maruyama, J.; Nagashima, Y.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in the xanthid crab Atergatis floridus. Nippon Suisan Gakkaishi 1983, 49, 1887–1892. [Google Scholar] [CrossRef]
- Sheumack, D.D.; Howden, M.E.; Spence, I.; Quinn, R.J. Maculotoxin: A neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189. [Google Scholar] [CrossRef]
- Hwang, D.-F.; Lu, S.C.; Jeng, S.-S. Occurrence of tetrodotoxin in the gastropods Rapana rapiformis and R. venosa venosa. Mar. Biol. 1991, 111, 65–69. [Google Scholar] [CrossRef]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef]
- Katikou, P. Public health risks associated with tetrodotoxin and its analogues in European waters: Recent advances after the EFSA scientific opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Jeon, J.K.; Maruyama, J.; Noguchi, T.; Ito, K.; Hashimoto, K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata (Platyhelminthes). Toxicon 1986, 24, 645–650. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 41, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V.J. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, H.; Itoi, S.; Sugita, H. TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan. Mar. Drugs 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Noguchi, T.; Hwang, D.F.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Vibrio alginolyticus, a tetrodotoxin-producing bacterium, in the intestine of the fish Fugu vermicularis vermicularis. Mar. Biol. 1987, 94, 625–630. [Google Scholar] [CrossRef]
- Simidu, U.; Noguchi, T.; Hwang, D.F.; Shida, Y.; Hashimoto, K. Marine bacteria which produce tetrodotoxin. Appl. Environ. Microbiol. 1987, 53, 1714–1715. [Google Scholar] [Green Version]
- Noguchi, T.; Arakawa, O. Tetrodotoxin – distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Matsui, T.; Hamada, S.; Konosu, S. Difference in accumulation of puffer fish toxin and crystalline tetrodotoxin in the puffer fish, Fugu rubripes rubripes. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 535–537. [Google Scholar] [CrossRef]
- Matsui, T.; Sato, H.; Hamada, S.; Shimizu, C. Comparison of toxicity of the cultured and wild puffer fish Fugu niphobles. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 253. [Google Scholar] [CrossRef]
- Honda, S.; Arakawa, O.; Takatani, T.; Tachibana, K.; Yagi, M.; Tanigawa, A.; Noguchi, T. Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi 2005, 71, 815–820. [Google Scholar] [CrossRef]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Commission of Japan. The liver of the aquacultured Japanese pufferfish (natural toxins): Summary. Food Saf. 2017, 5, 169–170. [Google Scholar] [CrossRef]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Itoi, S.; Kozaki, A.; Komori, K.; Tsunashima, T.; Noguchi, S.; Kawane, M.; Sugita, H. Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: Implications for TTX accumulation in the pufferfish. Toxicon 2015, 108, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Itoi, S.; Ueda, H.; Yamada, R.; Takei, M.; Sato, T.; Oshikiri, S.; Wajima, Y.; Ogata, R.; Oyama, H.; Shitto, T.; et al. Including planocerid flatworms in the diet effectively toxifies the pufferfish, Takifugu niphobles. Sci. Rep. 2018, 8, 12302. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Jeon, J.K.; Noguchi, T.; Ito, K.; Hashimoto, K. Distribution of tetrodotoxin in the tissues of the flatworm Planocera multitentaculata (Platyhelminthes). Toxicon 1987, 25, 975–980. [Google Scholar] [CrossRef]
- Yamada, R.; Tsunashima, T.; Takei, M.; Sato, T.; Wajima, Y.; Kawase, M.; Oshikiri, S.; Kajitani, Y.; Kosoba, K.; Ueda, H.; et al. Seasonal changes in the tetrodotoxin content of the flatworm Planocera multitentaculata. Mar. Drugs 2017, 15, 56. [Google Scholar] [CrossRef]
- Tsunashima, T.; Hagiya, M.; Yamada, R.; Koito, T.; Tsuyuki, N.; Izawa, S.; Kosoba, K.; Itoi, S.; Sugita, H. A molecular framework for the taxonomy and systematics of Japanese marine turbellarian flatworms (Platyhelminthes, Polycladida). Aquat. Biol. 2017, 26, 159–167. [Google Scholar] [CrossRef]
- Okita, K.; Yamazaki, H.; Sakiyama, K.; Yamane, H.; Niina, S.; Takatani, T.; Arakawa, O.; Sakakura, Y. Puffer smells tetrodotoxin. Ichthyol. Res. 2013, 60, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Itoi, S.; Yoshikawa, S.; Asahina, K.; Suzuki, M.; Ishizuka, K.; Takimoto, N.; Mitsuoka, R.; Yokoyama, N.; Detake, A.; Takayanagi, C.; et al. Larval pufferfish protected by maternal tetrodotoxin. Toxicon 2014, 78, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoi, S.; Suzuki, M.; Asahina, K.; Sawayama, E.; Nishikubo, J.; Oyama, H.; Takei, M.; Shiibashi, N.; Takatani, T.; Arakawa, O.; et al. Role of maternal tetrodotoxin in survival of larval pufferfish. Toxicon 2018, 148, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K. Tetrodotoxin as a pheromone. Nature 1995, 378, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Itoi, S.; Ishizuka, K.; Mitsuoka, R.; Takimoto, N.; Yokoyama, N.; Detake, A.; Takayanagi, C.; Yoshikawa, S.; Sugita, H. Seasonal changes in the tetrodotoxin content of the pufferfish Takifugu niphobles. Toxicon 2016, 114, 53–58. [Google Scholar] [CrossRef]
- Ishida, S. Embryogenesis in marine planarians (Polycladida). In Morpho-Differentiation in Planarians from Biological Basis to Gene Manipulation; Teshirogi, W., Watanabe, K., Eds.; Kyoritsu Shuppan Company: Tokyo, Japan, 1998; pp. 241–258. (In Japanese) [Google Scholar]

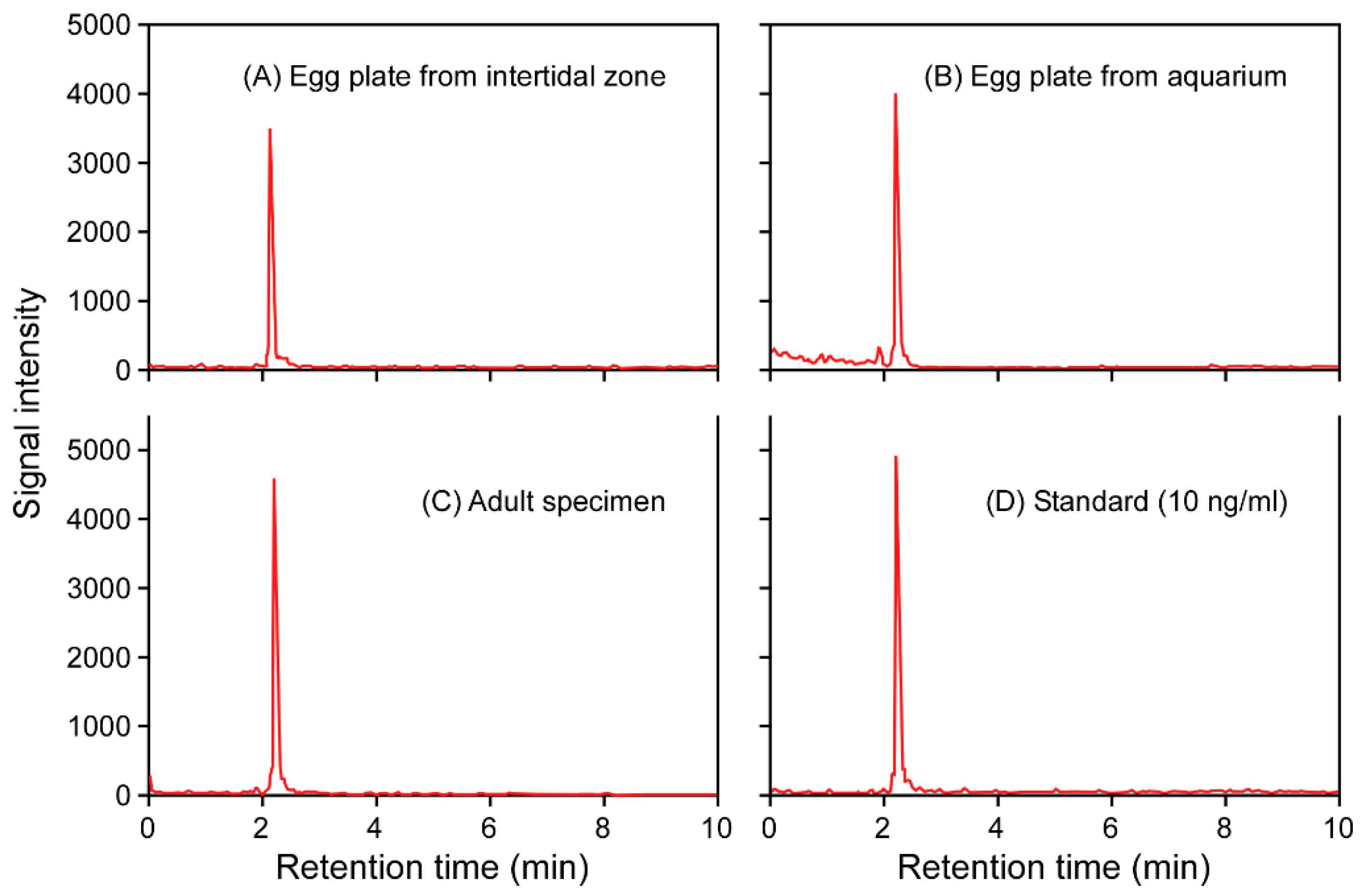

| Sample | n | Body Weight (g) | Sample from: | TTX Concentration (μg/g) | TTX Amount (μg) |
|---|---|---|---|---|---|
| Adult | 6 | 4.39 ± 0.92 | Wild | 115 ± 52 b | 523 ± 285 |
| Egg plate | 3 | N/A | Wild | 23 ± 16 a | N/A |
| Egg plate | 3 | N/A | Aquarium | 9328 ± 3356 a | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okabe, T.; Oyama, H.; Kashitani, M.; Ishimaru, Y.; Suo, R.; Sugita, H.; Itoi, S. Toxic Flatworm Egg Plates Serve as a Possible Source of Tetrodotoxin for Pufferfish. Toxins 2019, 11, 402. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070402
Okabe T, Oyama H, Kashitani M, Ishimaru Y, Suo R, Sugita H, Itoi S. Toxic Flatworm Egg Plates Serve as a Possible Source of Tetrodotoxin for Pufferfish. Toxins. 2019; 11(7):402. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070402
Chicago/Turabian StyleOkabe, Taiki, Hikaru Oyama, Maho Kashitani, Yuta Ishimaru, Rei Suo, Haruo Sugita, and Shiro Itoi. 2019. "Toxic Flatworm Egg Plates Serve as a Possible Source of Tetrodotoxin for Pufferfish" Toxins 11, no. 7: 402. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070402