Fire Ant Venom Alkaloids Inhibit Biofilm Formation
Abstract
:1. Introduction
2. Results
2.1. Solenopsins Extraction and Purification
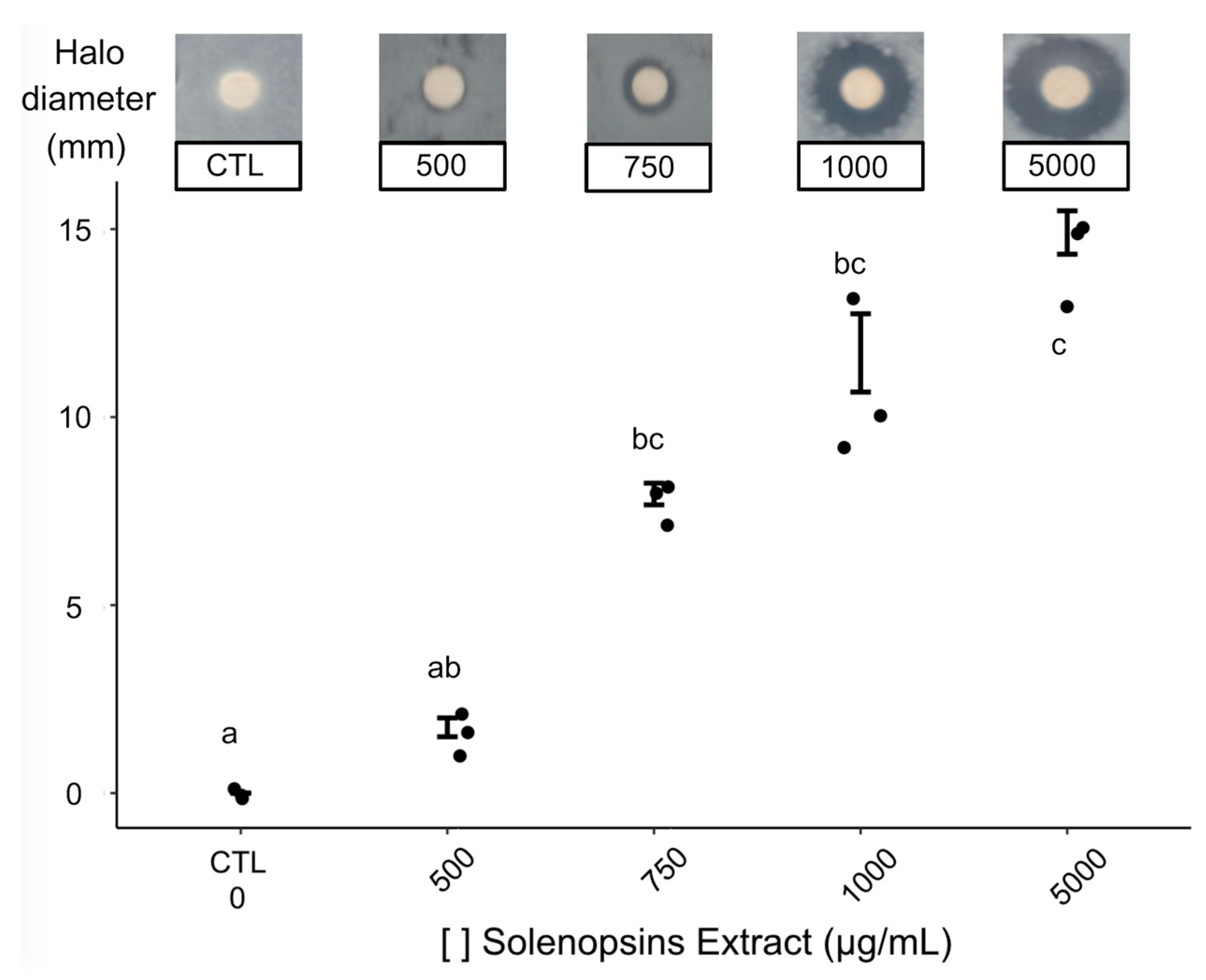
2.2. Antimicrobial Activity
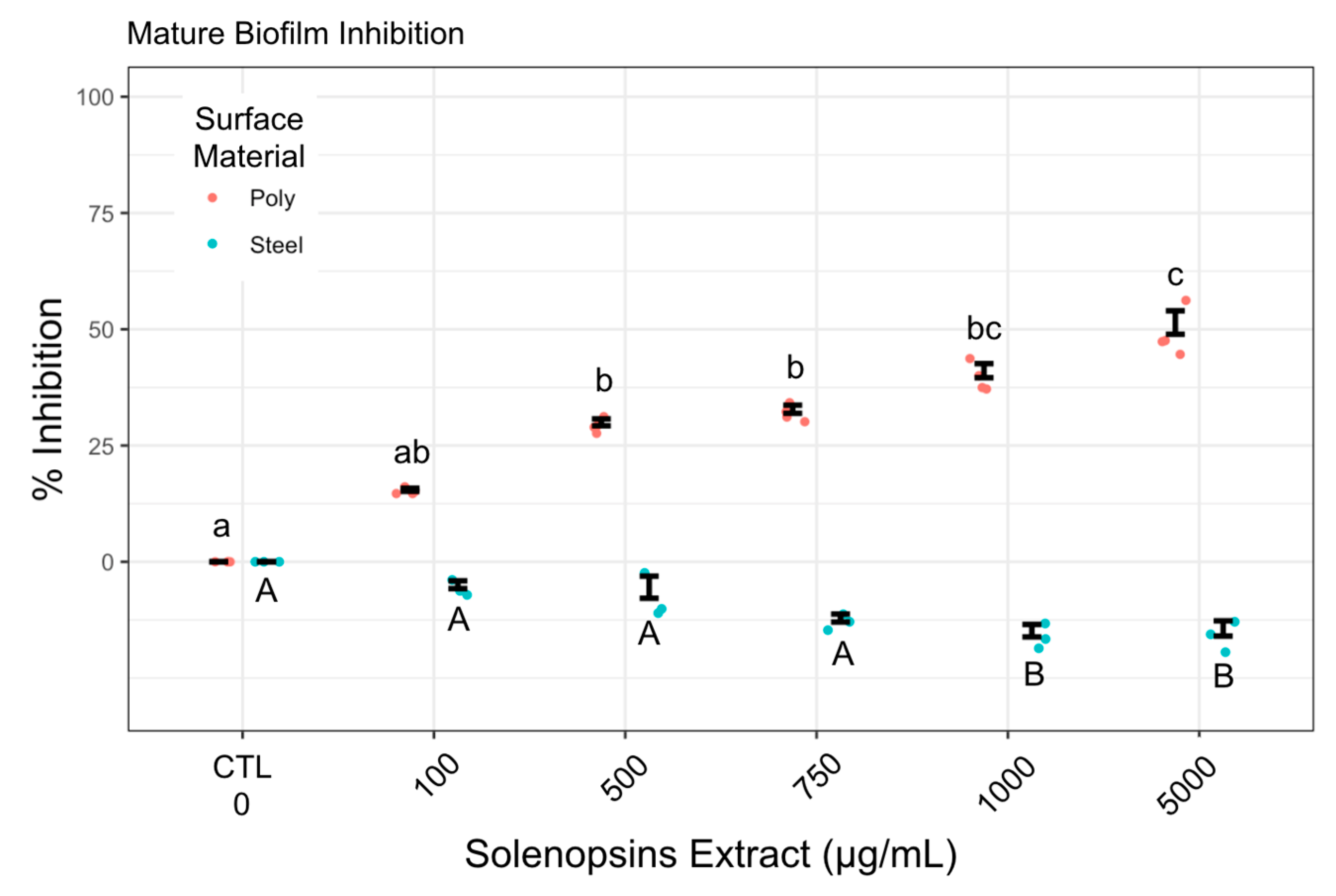
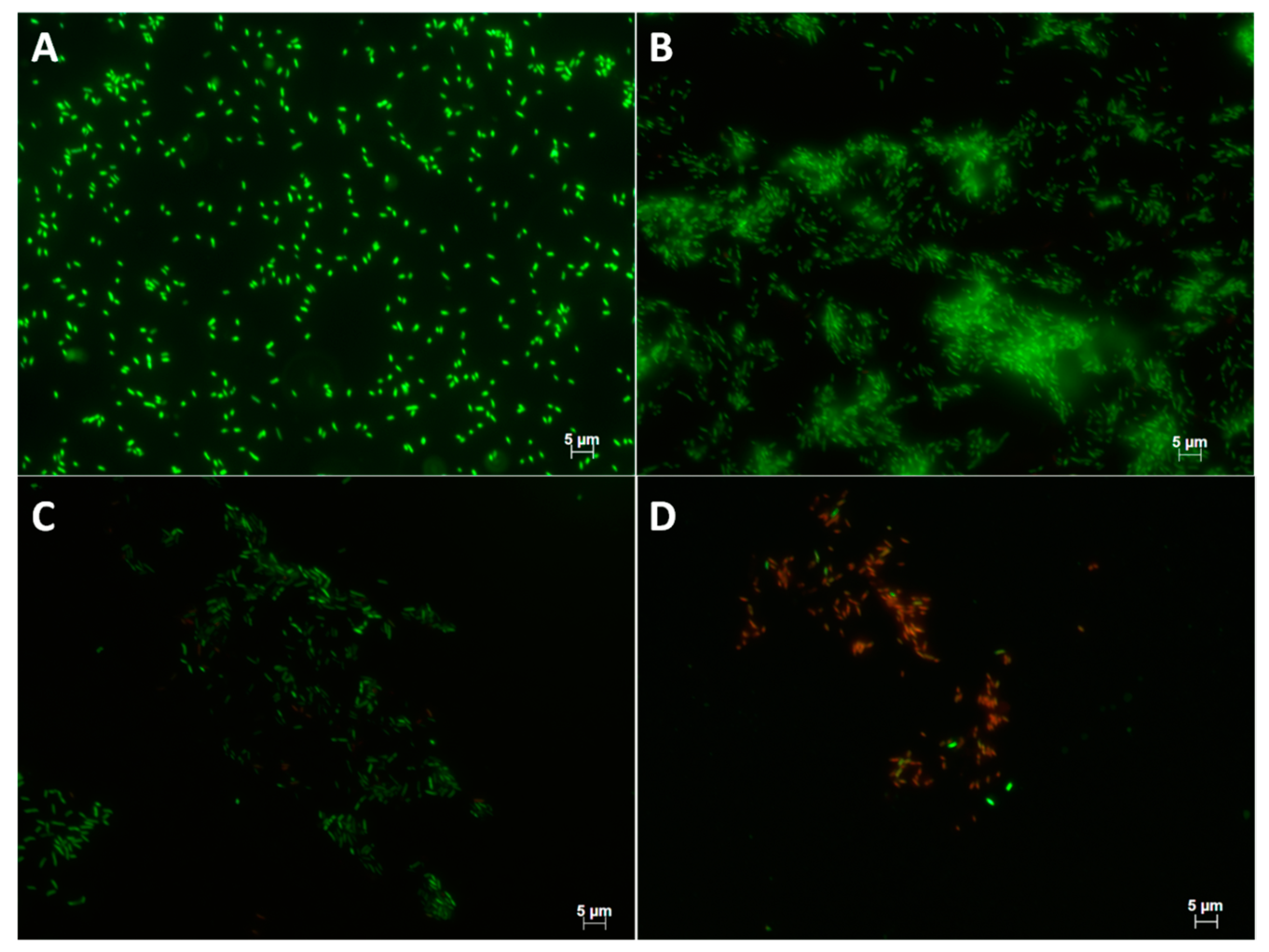
2.3. Effect of Preconditioning on Cell Adhesion and Biofilm Formation
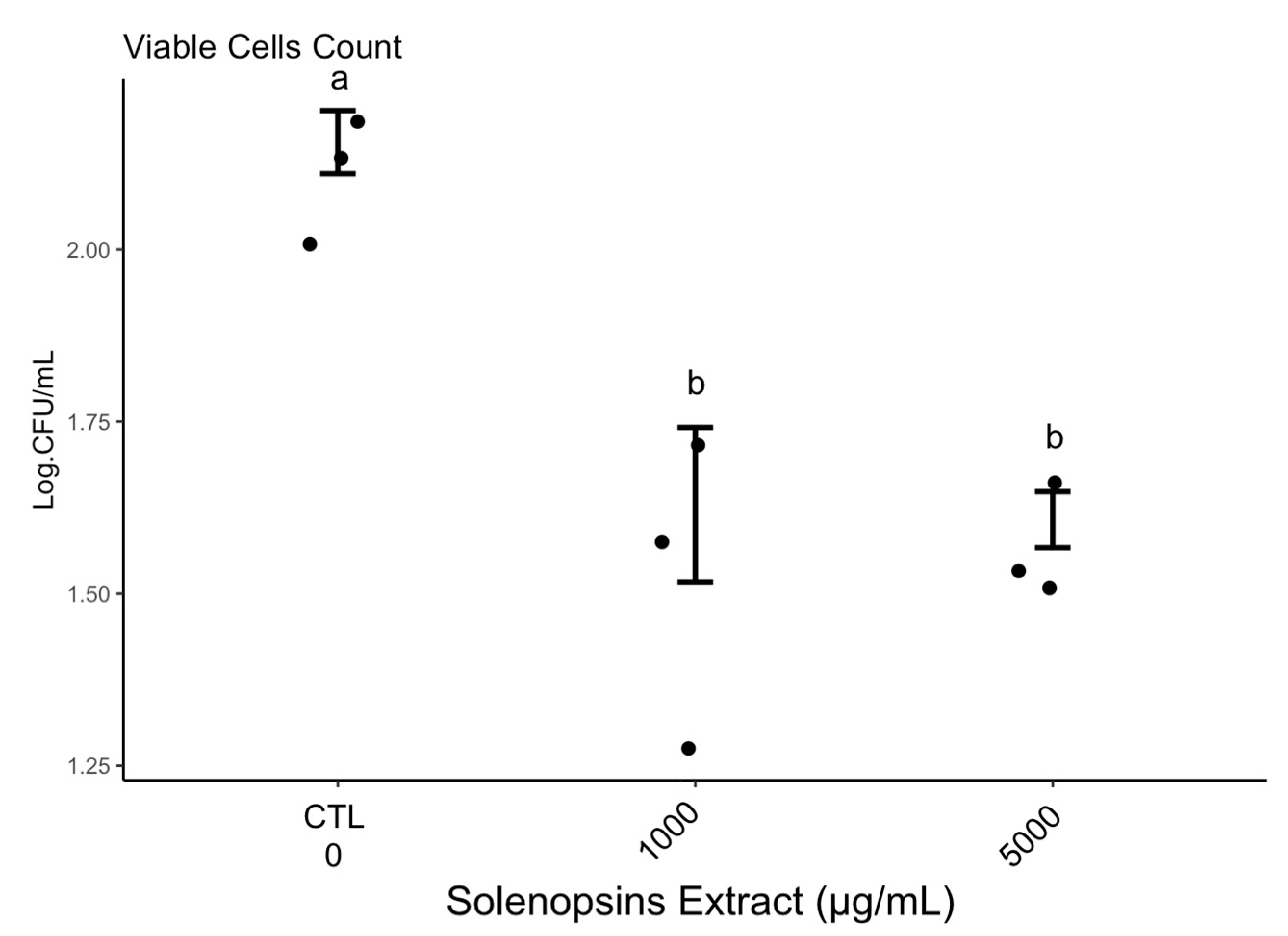
2.4. Quantification of Viable Cells
2.5. Physicochemical Properties: Surface Characteristics
3. Discussion
3.1. Compounds Production/Extraction
3.2. Antimicrobial Activity
3.3. Biofilm Formation and Suppression
3.4. Quantification of Viable Cells
3.5. Physico-Chemical Tests for Surfactant Chemistry
4. Conclusions
5. Materials and Methods
5.1. Solenopsins Extraction and Purification
5.2. Microbial Tests
5.2.1. Antimicrobial Activity
5.2.2. Quantification of Biofilm Formation
5.2.3. Effect of Surface Conditioning on Cell Adhesion
5.2.4. Effect of Surface Conditioning on Cell Viability
5.2.5. Epifluorescence Microscopy Observations
5.3. Physico-Chemical Tests for Surfactant Chemistry
5.3.1. Extraction of Biosurfactants (Positive Controls)
5.3.2. Physicochemical Properties
5.3.3. Surface Characteristics
5.3.4. Hydrophilic-Lipophilic Balance (HLB)
5.4. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jia, R.; Li, Y.; Gu, T. Advances in the treatment of problematic industrial biofilms. World J. Microbiol. Biotechnol. 2017, 33, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Costernon, J.W.; Irving, R.T.; Chen, K.J. The bacterial glycocalix in nature and disease. Annu. Rev. Microbiol. 1981, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. Inst. Pasteur 2003, 5, 1213–1219. [Google Scholar] [CrossRef]
- Hood, S.K.; Zottola, E. Biofilms in food processing. Food Control. Oxf. 1995, 6, 9–18. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lappin-Scott, H.M. Biofilms adhere to stay. Trends Microbiol. 2001, 9, 9–10. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Klosowska-Chomiczewska, I.; Medrzycka, K.; Karpenko, E. Biosurfactants—Biodegradability, toxicity, efficiency in comparison with synthetic surfactants. Adv. Chem. Mech. Eng. 2011, 2, 1–9. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.; Weilmeier, D.; Dineen, S.S.; Ralyea, R.; Boor, K.J. Molecular and Phenotypic characterization of Pseudomonas spisolated from milk. Appl. Environ. Microbiol. 2000, 66, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant. Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Mattila-Sandholm, T.; Wirtanen, G. Biofilm formation in the industry: A review. Food Rev. Int. 1992, 8, 573–603. [Google Scholar] [CrossRef]
- Araujo, L.V.; Freire, D.M.G.; Nitschke, M. Biossurfactantes: Propriedades anticorrosivas, antibiofilmes e antimicrobianas. Quim. Nova 2013, 36, 848–858. [Google Scholar] [CrossRef]
- Araujo, L.V.; Abreu, F.; Lins, U.; Anna, L.M.M.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin inhibit Listeria monocytogenes adhesion. Food Res. Int. 2011, 44, 481–488. [Google Scholar] [CrossRef]
- Nitschke, M.; Araújo, V.; Costa, S.G.V.A.O.; Pires, R.C.; Zeraik, A.E.; Fernandes, A.C.L.B.; Freire, D.M.G.; Contiero, J. Surfactin reduces the adhesion of food-borne pathogenic bacteria to solid surface. Lett. Appl. Microbiol. 2009, 49, 241–247. [Google Scholar] [CrossRef]
- Fox, E.G. Venom toxins of fire ants. In Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–16. [Google Scholar]
- Park, J.; Kaufmann, G.F.; Bowen, J.P.; Arbiser, J.L.; Janda, K.D. Solenopsin A, a venom alkaloid from the fire ant Solenopsis invicta, inhibits quorum sensing signalling in Pseudomonas aeruginosa. J. Infect. Dis. 2008, 198, 1198–1201. [Google Scholar] [CrossRef]
- Andrade, N.J. Higienização na indústria de alimentos: Avaliação e controle da adesão e formação de biofilmes bacterianos. In Higienização na Indústria de Alimentos: Avaliação e Controle da Adesão e Formação de Biofilmes Bacterianos; Livraria Varela: São Paulo, Brazil, 2008. [Google Scholar]
- Dufour, M.; Simmonds, R.S.; Bremer, J. Development of a laboratory scale clean-in-place system to test the effectiveness of ‘‘natural’’ antimicrobials against dairy biofilms. J. Food Prot. 2004, 67, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J.; Fillery, S.; McQuillan, A.J. Laboratory scale clean-in-place (CIP) studies on the effectiveness of different caustic and acid wash steps on the removal of dairy biofilms. Int. J. Food Microbiol. 2006, 106, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Huigens, R.W.; Rogers, S.A.; Steinhauer, A.T.; Melander, C. Inhibition of Acinetobacter baumannii, Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation with a class of TAGE-triazole conjugates. Org. Biomol. Chem. 2009, 7, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, X.; Zhu, Z.; Tang, T.; Dai, K.; Sadovskaya, I.; Jabbouri, S. Effect of berberine on Staphylococcus epidermidis biofilm formation. Int. J. Antimicrob. Agents 2009, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.S.; Naik, D.; Bhat, C.; Tilve, S.; Tilvi, S.; D’Souza, L. Synthesis of (R)-norbgugaine and its potential as quorum sensing inhibitor against Pseudomonas aeruginosa. Bioorganic Med. Chem. Lett. 2013, 23, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Furlani, R.E.; Yeagley, A.A.; Melander, C. A flexible approach to 1,4-di-substituted 2-aminoimidazoles that inhibit and disperse biofilms and potentiate the effects of ß-lactams against multi-drug resistant bacteria. Eur. J. Med. Chem. 2013, 62, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Kujala, J.; Busygin, I.; Leino, R.; Vuorela, P.M.; Fallarero, A. Evaluation of antibacterial and antibiofilm activities of cinchona alkaloid derivatives against Staphylococcus aureus. Nat. Prod. Commun. 2012, 7, 1173–1176. [Google Scholar]
- Li, S.; Jin, X.; Chen, J.; Lu, S. Inhibitory activities of venom alkaloids of Red Imported Fire Ant against Clavibacter michiganensis subsmichiganensis in vitro and the application of piperidine alkaloids to manage symptom development of bacterial canker on tomato in the greenhouse. Int. J. Pest. Manag. 2013, 59, 150–156. [Google Scholar] [CrossRef]
- Jouvenaz, D.P.; Blum, M.S.; MacConnell, J.G. Antibacterial activity of venom alkaloids from the imported fire ant, Solenopsin invicta Buren. Antimicrob. Agents Chemother. 1972, 2, 291–293. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Solis, D.R.; Santos, L.D.; Pinto, J.R.A.S.; Menegasso, A.R.S.; Silva, R.C.M.C.; Palma, M.S.; Bueno, O.C.; Machado, E.A. A simple, rapid method for the extraction of whole fire ant venom (Insecta: Formicidae: Solenopsis). Toxicon 2013, 65, 5–8. [Google Scholar]
- Fox, E.G.P. Chemical blueprints to identifying fire ants: Overview on venom alkaloids. BioRxiv 2018, 2018, 407775. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Pianaro, A.; Solis, D.R.; Delabie, J.H.C.; Vairo, B.C.; MacHado, E.D.A.; Bueno, O.C. Intraspecific and intracolonial variation in the profile of venom alkaloids and cuticular hydrocarbons of the fire ant Solenopsis saevissima Smith (Hymenoptera: Formicidae). Psyche J. Entomol. 2012, 2012, 398061. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Xu, M.; Wang, L.; Chen, L.; Lu, Y.Y. Speedy milking of fresh venom from aculeate hymenopterans. Toxicon 2019, 146, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-H.; Hu, L.; Wang, W.-K.; Vander Meer, R.K.; Porter, S.D.; Chen, L. Workers and alate queens of Solenopsis geminata share qualitatively similar but quantitatively different venom alkaloid chemistry. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef]
- IUCN. View 100 of the World’s Worst Invasive Alien Species [EB/OL]. Available online: http://www.issg.org/worst100_species.html (accessed on 6 May 2019).
- Sullivan, D.C.; Flowers, H.; Rockhold, R.; Herath, H.M.T.B.; Nanayakkara, N.P.D. Antibacterial activity of synthetic fire ant venom: The solenopsins and isosolenopsins. Am. J. Med. Sci. 2009, 338, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lind, N.K. Mechanisms of action of fire ant (Solenopsis) venom I. Lytic release histamine from mast cells. Toxicon 1982, 20, 831–840. [Google Scholar] [CrossRef]
- Hullin, V.; Mathat, A.G.; Mafart, P.; Dufosé, L. Les proprietés anti-microbiennes des huiles essentielles et composés d’arômes. Sci. Des. Aliment. 1998, 18, 563–582. [Google Scholar]
- Robbers, J.E.; Speedie, M.K.; Tyler, V.E. Farmacognosia & Farmacobiotecnologia; Editora Premier: São Paulo, Brazil, 1997. [Google Scholar]
- Pereira, J.V.; Pereira, M.S.V.; Sampaio, F.C.; Sampaio, M.C.C.; Alves, P.M.; Araujo, C.R.F.; Higino, J.S. Efeito antibacteriano e antiaderente in vitro do extrato da Punica granatum Linn. sobre microrganismos do biofilme dental. Braz. J. Pharmacogn. 2006, 16, 88–93. [Google Scholar] [CrossRef]
- Mclandsborough, L.; Rodriguez, A.; Perez-Conesa, D.; Weiss, J. Biofilms: At the interface between biophysics and microbiology. Food Biophys. 2006, 1, 94–114. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surface. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Van Oss, C.J. Hydrophobicity of biosurfaces origin, quantitative determination and interaction energies. Colloids Surf. B Biointerfaces 1995, 5, 91–110. [Google Scholar] [CrossRef]
- Araujo, E.A.; Andrade, N.J.; Carvalho, A.F.; Ramos, A.M.; Silva, C.A.S.; Silva, L.H.M. Aspectos coloidais da adesão de microrganismos. Quim. Nova 2009, 33, 1940–1948. [Google Scholar] [CrossRef]
- Gomes, M.Z.V.; Nitschke, M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control. 2012, 25, 441–447. [Google Scholar] [CrossRef]
- Araujo, L.V.; Guimarães, C.R.; Marquita, R.L.S.; Santiago, V.M.J.; Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control. 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Pitts, J.P.; McHugh, J.V.; Ross, K.G. Cladistic analysis of the fire ants of the Solenopsis saevissima species-group (Hymenoptera: Formicidae). Zool. Scr. 2005, 34, 493–505. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Solis, D.R.; Lazoski, C.; Mackay, W. Weaving through a cryptic species: Comparing the Neotropical ants Camponotus senex and Camponotus textor (Hymenoptera: Formicidae). Micron 2017, 99, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Screening and partial characterization of Bacillus with potential applications in biocontrol of cucumber Fusarium wilt. Crop. Prot. Amst. 2012, 35, 29–35. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabic-Vlahovic, M. Biofilm formation by Salmonella spand Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods Columbia 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N.; Karpenko, E.; Shulga, A. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Sampaio, A.P.; Vasquez, G.S.; Santa Anna, L.M.; Freire, D.M.G. Evaluation of different carbon in nitrogen sources in production of rhamnolipids by a strain of Pseudomonas aeruginosa. Appl. Biochem. Biotechnol. 2002, 98, 1025–1035. [Google Scholar] [CrossRef]
- Song, B.; Springer, J. Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing. J. Colloid Interface Sci. 1996, 184, 64–76. [Google Scholar] [PubMed]
- Sheppard, J.D.; Mulligan, C.N. The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl. Microbiol. Biotechnol. 1987, 27, 110–116. [Google Scholar] [CrossRef]
- Van Oss, C.J.M.; Chaudhury, I.C.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]

| Compound Name | Short Trivial Name | Chemical Formula | Diagnostic Ions-m/z | RT (Initial) | Relative Abundance (Area %) |
|---|---|---|---|---|---|
| cis-2-Me-6-Tridecyl-Piperidine | C13 | C19H39N | 280 (M+), 266, 98 | 19.942 | 2.594 |
| trans-2-Me-6-Tridecenyl-Piperidine | C13:1 | C19H38N | 279 (M+), 264, 180, 124, 111, 98 | 20.167 | 76.528 |
| trans-2-Me-6-Tridecyl-Piperidine | C13 | C19H39N | 280 (M+), 266, 98 | 20.375 | 6.349 |
| cis-2-Me-6-Pentadecyl-Piperidine | C15 | C21H43N | 309 (M+), 308, 294, 98 | 21.550 | 0.394 |
| trans-2-Me-6-Pentadecenyl-Piperidine | C15:1 | C21H42N | 307 (M+), 292, 228, 154, 124, 111, 98 | 21.833 | 11.554 |
| trans-2-Me-6-Pentadecyl-Piperidine | C15 | C21H43N | 309 (M+), 308, 294, 98 | 22.025 | 2.580 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.B.d.; Fox, E.G.P.; Santos, D.G.d.; Sousa, J.S.d.; Freire, D.M.G.; Nogueira, F.C.S.; Domont, G.B.; Castilho, L.V.A.d.; Machado, E.d.A. Fire Ant Venom Alkaloids Inhibit Biofilm Formation. Toxins 2019, 11, 420. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070420
Carvalho DBd, Fox EGP, Santos DGd, Sousa JSd, Freire DMG, Nogueira FCS, Domont GB, Castilho LVAd, Machado EdA. Fire Ant Venom Alkaloids Inhibit Biofilm Formation. Toxins. 2019; 11(7):420. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070420
Chicago/Turabian StyleCarvalho, Danielle Bruno de, Eduardo Gonçalves Paterson Fox, Diogo Gama dos Santos, Joab Sampaio de Sousa, Denise Maria Guimarães Freire, Fabio C. S. Nogueira, Gilberto B. Domont, Livia Vieira Araujo de Castilho, and Ednildo de Alcântara Machado. 2019. "Fire Ant Venom Alkaloids Inhibit Biofilm Formation" Toxins 11, no. 7: 420. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11070420