Biological Control of Citrus Postharvest Phytopathogens
Abstract
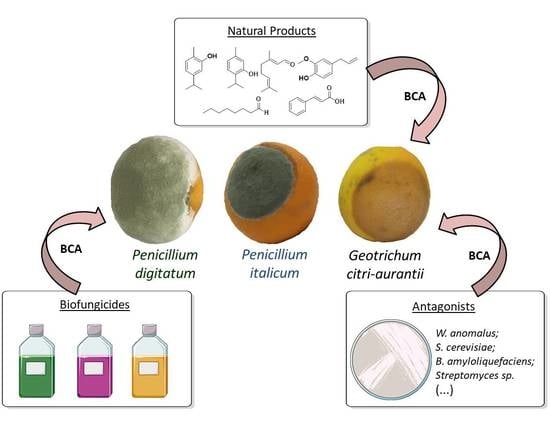
:1. Introduction
2. Alternative Control Methods
2.1. Microrganisms
2.2. Natural Products
2.3. Commercial Biofungicides
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ismail, M.; Zhang, J. Post–harvest citrus diseases and their control. Outlooks Pest Manag. 2004, 15, 29–35. [Google Scholar] [CrossRef]
- USDA/FAS. Citrus: World Markets and Trade. United States Department of Agriculture. Foreing Agricultural Service, February, p. 1–13, 2019. Available online: https://www.fas.usda.gov/data/citrus-world-markets-and-trade (accessed on 30 May 2019).
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants 2019, 8, 26. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.R.; Larsen, T.O.; Duus, J.Ø.; Barrero, A.F. Penicillium digitatum metabolites on synthetic media and citrus fruits. J. Agric. Food Chem. 2002, 50, 6361–6365. [Google Scholar] [CrossRef] [PubMed]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind. Crops Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Zhu, C.; Sheng, D.; Wu, X.; Wang, M.; Hu, X.; Li, H.; Yu, D. Identification of secondary metabolite biosynthetic gene clusters associated with the infection of citrus fruit by Penicillium digitatum. Postharvest Biol. Technol. 2017, 134, 17–21. [Google Scholar] [CrossRef]
- Barmore, C.R.; Brown, G.E. Polygalacturonase from citrus fruit infected with Penicillium italicum. Phytopathol. 1981, 71, 328–331. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Cohen, L.; Eick, A.; Rafael, G.; Belausov, E.; Wisniewski, M.; Droby, S. Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 2007, 97, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.P.; Cunha, T.; Silva, A.C.; Kupper, K.C. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 2016, 188, 72–79. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T. LED Blue light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017, 218, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Ben-Yehoshua, S.; Shapiro, B.; Henis, Y.; Carmeli, S. Accumulation of scoparone in heat-treated lemon fruit inoculated with Penicillium digitatum Sacc. Plant Physiol. 1991, 97, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Jeong, R.D.; Chu, E.H.; Lee, G.W.; Cho, C.; Park, H.J. Inhibitory effect of gamma irradiation and its application for control of postharvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 2016, 234, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Volentini, S.I.; Rapisarda, V.A. UVA photoactivation of harmol enhances its antifungal activity against the phytopathogens Penicillium digitatum and Botrytis cinerea. Front. Microbiol. 2017, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Usall, J.; Manso, T.; Torres, R.; Olmo, M.; García, J.M. Effect of high temperature treatments on growth of Penicillium spp. and their development on ‘Valencia’ oranges. Food Sci. Technol. Int. 2007, 13, 63–68. [Google Scholar] [CrossRef]
- Fatemi, S.; Borji, H. The effect of physical treatments on control of Penicillium digitatum decay orange cv. Valencia during storage period. Afr. J. Agric. Res. 2011, 6, 5757–5760. [Google Scholar] [CrossRef]
- Mulas, M. Combined effects of fungicides and thermotherapy on post-harvest quality of horticultural commodities. In Fungicides—Beneficial and Harmful Aspects; Thajuddin, N., Ed.; IntechOpen: London, UK, 2011; pp. 133–166. [Google Scholar]
- Abraham, A.O.; Laing, M.D.; Bower, J.P. Isolation and in vivo screening of yeast and Bacillus antagonists for the control of Penicillium digitatum of citrus fruit. Biol. Control 2010, 53, 32–38. [Google Scholar] [CrossRef]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Aoumar, A.A.B. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Mohammadi, P.; Tozlu, E.; Kotan, R.; Şenol Kotan, M. Potential of some bacteria for biological control of postharvest citrus green mould caused by Penicillium digitatum. Plant Protect. Sci. 2017, 53, 134–143. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Fallanaj, F.; Ippolito, A.; Ligorio, A.; Garganese, F.; Zavanella, C.; Sanzani, S.M. Electrolyzed sodium bicarbonate inhibits Penicillium digitatum and induces defence responses against green mould in citrus fruit. Postharvest Biol. Technol. 2016, 115, 18–29. [Google Scholar] [CrossRef]
- Shi, S.; Wang, F.; Lu, Y.; Deng, J. Combination of chitosan and salicylic acid to control postharvest green mold caused by Penicillium digitatum in grapefruit fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Nunes, C.A.; Manso, T.; Lima-Costa, M.E. Postharvest biological control of citrus fruits. Tree For. Sci. Biotechnol. 2009, 2, 116–126. [Google Scholar]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakul, W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Droby, S.; Chalutz, E.; Wilson, C.L.; Wisniewski, M. Characterization of the biocontrol activity of Debaryomyces hansenii in the control of Penicillium digitatum on grapefruit. Can. J. Microbiol. 1989, 35, 794–800. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, H.; Wu, C.; Fang, W.; Yu, C.; Ye, C.; Shi, Y.; Yu, T.; Zheng, X. Rhodosporidium paludigenum induces resistance and defense-related responses against Penicillium digitatum in citrus fruit. Postharvest Biol. Technol. 2013, 85, 196–202. [Google Scholar] [CrossRef]
- Bar-Shimon, M.; Yehuda, H.; Cohen, L.; Weiss, B.; Kobeshnikov, A.; Daus, A. Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr. Genet. 2004, 45, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Droby, S.; Bauchan, G.; Wisniewski, M. Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: A new role for reactive oxygen species in postharvest biocontrol? Postharvest Biol. Technol. 2010, 58, 194–202. [Google Scholar] [CrossRef]
- Castoria, R.; Caputo, L.; De Curtis, F.; De Cicco, V. Resistance of postharvest biocontrol yeasts to oxidative stress: A possible new mechanism of action. Phytopathology 2003, 93, 564–572. [Google Scholar] [CrossRef]
- Benhamou, N. Potential of the mycoparasite, Verticillium lecanii, to protect citrus fruit against Penicillium digitatum, the causal agent of green mold: A comparison with the effect of chitosan. Phytopathology 2004, 94, 693–705. [Google Scholar] [CrossRef]
- Aloui, H.; Licciardello, F.; Khwaldia, K.; Hamdi, M.; Restuccia, C. Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int. J. Food Microbiol. 2015, 200, 22–30. [Google Scholar] [CrossRef]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef]
- Comitini, F.; Mannazzu, I.; Ciani, M. Tetrapisispora phaffii killer toxin is a highly specific β-glucanase that disrupts the integrity of the yeast cell wall. Microb. Cell Fact. 2009, 8, 55. [Google Scholar] [CrossRef]
- Perez, M.F.; Contreras, L.; Garnica, N.M.; Fernández-Zenoff, M.V.; Farías, M.E.; Sepulveda, M.; Ramallo, J.; Dib, J.R. Native killer yeasts as biocontrol agents of postharvest fungal diseases in lemons. PLoS ONE 2016, 11, e0165590. [Google Scholar] [CrossRef]
- Kupper, K.C.; Cervantes, A.L.L.; Klein, M.N.; Silva, A.C. Avaliação de microrganismos antagônicos, Saccharomyces cerevisiae e Bacillus subtilis para o controle de Penicillium digitatum. Rev. Bras. Frutic. 2013, 35, 425–436. [Google Scholar] [CrossRef]
- Cunha, T.; Ferraz, L.P.; Wehr, P.P.; Kupper, K.C. Antifungal activity and action mechanisms of yeasts isolates from citrus against Penicillium italicum. Int. J. Food Microbiol. 2018, 276, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Leelasuphakul, W.; Hemmanee, P.; Chuenchitt, S. Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol. Technol. 2008, 48, 113–121. [Google Scholar] [CrossRef]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M.E. Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 2017, 63, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Yan, Y.H.; Wang, J.M.; Zhang, H.P.; Qi, W. Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Cornea, C.P.; Matei, S.; Matei, G.M.; Rodino, S. Comparative antifungal effect of lactic acid bacteria strains on Penicillium digitatum. Bull. UASVM Food Sci. Technol. 2015, 72, 226–230. [Google Scholar] [CrossRef]
- Maldonado, M.C.; Orosco, C.E.; Gordillo, M.A.; Navarro, A.R. In vivo and in vitro antagonism of Streptomyces sp. RO3 against Penicillium digitatum and Geotrichum candidum. Afr. J. Microbiol. Res. 2010, 4, 2451–2456. [Google Scholar]
- Najmeh, S.; Hosein, S.B.G.; Sareh, S.; Bonjar, L.S. Biological control of citrus green mould, Penicillium digitatum, by antifungal activities of Streptomyces isolates from agricultural soils. Afr. J. Microbiol. Res. 2014, 8, 1501–1509. [Google Scholar] [CrossRef]
- Walling, L.L. Induced resistance: From the basic to the applied. Trends Plant Sci. 2001, 6, 445–447. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. The effect of locust bean gum (LBG)-based edible coatings carrying biocontrol yeasts against Penicillium digitatum and Penicillium italicum causal agents of postharvest decay of mandarin fruit. Food Microbiol. 2016, 58, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, S.; Deng, L.; Ming, J.; Zeng, K. Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 2019, 152, 100–110. [Google Scholar] [CrossRef]
- Pimenta, R.S.; Silva, J.F.M.; Coelho, C.M.; Morais, P.B.; Rosa, C.A.; Corrêa, A., Jr. Integrated control of Penicillium digitatum by the predacious yeast Saccharomycopsis crataegensis and sodium bicarbonate on oranges. Braz. J. Microbiol. 2010, 41, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Chen, S.; Hu, M.; Haq, M.R.; Lai, K.; Qu, F.; Zhang, Y. Combination of Kluyveromyces marxianus and sodium bicarbonate for controlling green mold of citrus fruit. Int. J. Food Microbiol. 2011, 151, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, S.; Zeng, L.; Zheng, X.; Yu, T. Rhodosporidium paludigenum induced resistance in Ponkan mandarin against Penicillium digitatum requires ethylene-dependent signaling pathway. Postharvest Biol. Technol. 2014, 97, 93–101. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Unraveling the mechanisms used by antagonistic yeast to control postharvest pathogens on fruit. Acta Hortic. 2016, 1144, 63–70. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- Chalutz, E. Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii. Plant Dis. 1990, 74, 134–137. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Ochoa, J.L.; Troyo-Diéguez, E.; Larralde-Corona, C.P. Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 2010, 56, 181–187. [Google Scholar] [CrossRef]
- Choudhary, B.; Nagpure, A.; Gupta, R.K. Biological control of toxigenic citrus and papaya-rotting fungi by Streptomyces violascens MT7 and its extracellular metabolites. J. Basic Microbiol. 2015, 55, 1343–1356. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef]
- Lu, L.; Yang, Y.L.J.; Azat, R.; Yu, T.; Zheng, X. Quaternary chitosan oligomers enhance resistance and biocontrol efficacy of Rhodosporidium paludigenum to green mold in satsuma orange. Carbohydr. Polym. 2014, 113, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.H.; Salem, M.F.; Mazrou, K.E.; El-Tras, W.F. Control of citrus molds using bioactive coatings incorporated with fungal chitosan/plant extracts composite. J. Sci. Food Agric. 2016, 96, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- El Guilli, M.; Hamza, A.; Clément, C.; Ibriz, M.; Barka, E.A. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 2016, 6, 12. [Google Scholar] [CrossRef]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Tao, N.; Jia, L.; He, X. Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol. Technol. 2014, 90, 52–55. [Google Scholar] [CrossRef]
- Wu, Y.; OuYang, Q.; Tao, N. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Solgi, M.; Ghorbanpour, M. Application of essential oils and their biological effects on extending the shelf-life and quality of horticultural crops. Trakia J. Sci. 2014, 12, 198–210. [Google Scholar]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Trabelsi, D.; Hamdane, A.M.; Said, M.B.; Abdrrabba, M. Chemical composition and antifungal activity of essential oils from flowers, leaves and peels of Tunisian Citrus aurantium against Penicillium digitatum and Penicillium italicum. J. Essent. Oil Bear Plants 2016, 19, 1660–1674. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H.; He, X. Effect of octanal on the mycelial growth of Penicillium italicum and P. digitatum. World J. Microbiol. Biotechnol. 2014, 30, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Kanan, G.J.M.; Al-Najar, R.A.-W.K. In vitro and in vivo activity of selected plant crude extracts and fractions against Penicillium italicum. J. Plant Prot. Res. 2009, 49, 341–352. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Bayat, H. Antifungal activity of black caraway and anise essential oils against Penicillium digitatum on blood orange fruits. Int. J. Fruit Sci. 2018, 18, 307–319. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Li, D.; Xia, H.; Su, X.; Peng, L.; Pan, S. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016, 196, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, P.; Chen, C.; Peng, X.; Li, M.; Chen, M.; Wang, J.; Chen, J. Antifungal activity of Ramulus cinnamomi explored by 1H-NMR based metabolomics approach. Molecules 2017, 22, 2237. [Google Scholar] [CrossRef]
- Hendel, N.; Larous, L.; Belbey, L. Antioxidant activity of rosemary (Rosmarinus officinalis L.) and its in vitro inhibitory effect on Penicillium digitatum. Int. Food Res. J. 2016, 23, 1725–1732. [Google Scholar]
- Exarchou, V.; Kanetis, L.; Charalambous, Z.; Apers, S.; Pieters, L.; Gekas, V.; Goulas, V. HPLC-SPE-NMR Characterization of major metabolites in Salvia fruticosa Mill. extract with antifungal potential: Relevance of carnosic acid, carnosol, and hispidulin. J. Agric. Food Chem. 2015, 63, 457–463. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Musto, M.; Potenza, G.; Cellini, F. Inhibition of Penicillium digitatum by a crude extract from Solanum nigrum leaves. Biotechnol. Agron. Soc. Environ. 2014, 18, 174–180. [Google Scholar]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Aoumar, A.A.B. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Ameziane, N.; Boubaker, H.; Boudyach, H.; Msanda, F.; Jilal, A.; Benaoumar, A.A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 2007, 27, 273–277. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. 2013, 41, 86–92. [Google Scholar] [CrossRef]
- Mekbib, S.B.; Regnier, T.J.C.; Korsten, L. Control of Penicillium digitatum growth on citrus fruit using two plant extracts and their mode of action. Phytoparasitica 2007, 35, 264–276. [Google Scholar] [CrossRef]
- Obagwu, J.; Korsten, L. Control of citrus green and blue molds with garlic extracts. Eur. J. Plant Pathol. 2003, 109, 221–225. [Google Scholar] [CrossRef]
- Mossini, S.A.G.; Arrotéia, C.C.; Kemmelmeier, C. Effect of neem leaf extract and neem oil on Penicillium growth, sporulation, morphology and Ochratoxin A production. Toxins 2009, 1, 3–13. [Google Scholar] [CrossRef]
- Baviskar, R.N. Anti-fungal activity of Launea pinnatifida and Argimone maxicana against post-harvest fungal pathogens in Apple fruits. Int. J. of Life Sciences 2014, 2, 346–349. [Google Scholar]
- Singh, H.; Al-samarai, G.; Syarhabil, M. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of citrus. IJSR 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Palou, L. Penicillium digitatum, Penicillium italicum (Green Mold, Blue Mold). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 45–102. [Google Scholar]
- Holmes, G.J.; Eckert, J.W. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathol. 1999, 89, 716–721. [Google Scholar] [CrossRef]
- Du Plooy, W.; Regnier, T.; Combrinck, S. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 2009, 53, 117–122. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K.; Shukla, A.K. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J. Microbiol. Biotechnol. 2008, 24, 39–46. [Google Scholar] [CrossRef]
- Fawcett, C.H.; Spencer, D.M. Plant chemotherapy with natural products. Annu. Rev. Phytopathol. 1970, 8, 403–418. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Aoumar, A.A.B. In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Prot. 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Jham, G.N.; Dhingra, O.D.; Jardim, C.M.; Valente, V.M.M. Identification of the major fungitoxic component of cinnamon bark oil. Fitopatol. Bras. 2005, 30, 404–408. [Google Scholar] [CrossRef]
- Telezhenetskaya, M.V.; D’yakonov, A.L. Alkaloids of Peganum harmala. Unusual reaction of peganine and vasicinone. Chem. Nat. Compd. 1991, 27, 471–474. [Google Scholar] [CrossRef]
- Scora, K.M.; Scora, R.W. Effect of volatiles on mycelium growth of Penicillium digitatum, P. italicum, and P. ulaiense. J. Basic Microbiol. 1998, 38, 405–413. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Mercier, J.; Smilanick, J.L. Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol. Control 2005, 32, 401–407. [Google Scholar] [CrossRef]
- Nakamura, M.; Suprapta, D.N.; Iwai, H.; Arai, A.K. Comparison of endo-polygalacturonase activities of citrus and non-citrus races of Geotrichum candidum, and cloning and expression of the corresponding genes. Mol. Plant Pathol. 2001, 2, 265–274. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.H.; Förster, H.; Adaskaveg, J.E. Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Liu, H.; Shan, Y.; Gupta, V.K.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B as a biological preservative: Purification, fungicidal activity and mechanism of action against Geotrichum citri-aurantii. Biomolecules 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Aoumar, A.A.B. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett. Appl. Microbiol. 2012, 55, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.; Boubaker, H.; Askarne, L.; Talibi, I.; Msanda, F.; Boudyach, E.H.; Saadi, B.; Aoumar, A.A.B. Antifungal properties of organic extracts of eight Cistus L. species against postharvest citrus sour rot. Lett. Appl. Microbiol. 2015, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sayago, J.E.; Ordoñez, R.M.; Kovacevich, L.N.; Torres, S.; Isla, M.I. Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol. Technol. 2012, 67, 19–24. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.P.; Li, Y.C.; Yu, T.; Zheng, X.D. Antifungal activity of thyme oil against Geotrichum citri-aurantii in vitro and in vivo. J. Appl. Microbiol. 2009, 107, 1450–1456. [Google Scholar] [CrossRef]
- Xu, S.-X.; Li, Y.-C.; Liu, X.; Mao, L.-J.; Zhang, H.; Zheng, X.-D. In vitro and in vivo antifungal activity of a water-dilutable cassia oil microemulsion against Geotrichum citri-aurantii. J. Sci. Food Agric. 2012, 92, 2668–2671. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Alamri, S.A. The synergistic effect of two formulated biofungicides in the biocontrol of root and bottom rot of lettuce. Biocontrol Sci. 2014, 19, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Jeffers, S.N. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in cold storage. Crop Prot. 1997, 16, 629–633. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 241–262. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts tomanage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zamanizadeh, H.R.; Hatami, N.; Aminaee, M.M.; Rakhshandehroo, F. Application of biofungicides in control of damping disease off in greenhouse crops as a possible substitute to synthetic fungicides. Int. J. Environ. Sci. Technol. 2011, 8, 129–136. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Mari, M.; Di Francesco, A.; Bertolini, P. Control of fruit postharvest diseases: Old issues and innovative approaches. Stewart Postharvest Rev. 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Anuagasi, C.L.; Okigbo, R.N.; Anukwuorji, C.A.; Okereke, C.N. The impact of biofungicides on agricultural yields and food security in Africa. IJAT 2017, 13, 953–978. [Google Scholar]
- Piombo, E.; Sela, N.; Wisniewski, M.; Hoffmann, M.; Gullino, M.L.; Allard, M.W.; Levin, E.; Spadaro, D.; Droby, S. Genome sequence, assembly and characterization of two Metschnikowia fructicola strains used as biocontrol agents of postharvest diseases. Front. Microbiol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Wisniewski, M.; Macarisin, D.; Droby, S. Challenges and opportunities for the commercialization of postharvest biocontrol. In Proceedings of the VI International Postharvest Symposium, Antalya, Turkey, 8–12 April 2009; International Society for Horticultural Science: Leuven, Belgium, 2010; Volume 877, pp. 1577–1582. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Droby, S. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 2001, 24, 395–399. [Google Scholar] [CrossRef]
- Viñas, I.; Usall, J.; Teixidó, N.; Sanchis, V. Biological control of major postharvest pathogens on apple with Candida sake. Int. J. Food Microbiol. 1998, 40, 9–16. [Google Scholar] [CrossRef]
- Fravel, D.R.; Larkin, R.P. Availability and application of biocontrol products. In Biological and Cultural Tests for Control of Plant Diseases; Canaday, C.H., Ed.; APS Press: St. Paul, MN, USA, 1996; Volume 11, pp. 1–7. [Google Scholar]
- Souza, J.R.B.; Kupper, K.C.; Augusto, F. In vivo investigation of the volatile metabolome of antiphytopathogenic yeast strains active against Penicillium digitatum using comprehensive two-dimensional gas chromatography and multivariate data analysis. Microchem. J. 2018, 141, 204–209. [Google Scholar] [CrossRef]




| Antagonist | Agent | Mechanism | Target Pathogen | References |
|---|---|---|---|---|
| Yeast | Wickerhamomyces anomalus (or Pichia anomala) | Antibiosis, competition for nutrients, fruit resistance induction and ‘killer’ activity | P. digitatum P. italicum | [39,40,41,52] |
| Saccharomyces cerevisiae | Competition for nutrients or space and ‘killer’ activity | P. digitatum P. italicum | [40,41,43,44] | |
| Candida oleophila | Resistance induction. Increase phenylalanine ammonia lyase activity and accumulation of the phytoalexins such as scoparone, scopoletin, and umbelliferone | P. digitatum P. italicum | [33,53] | |
| Saccharomycopsis crataegensis + sodium bicarbonate | Not specified | P. digitatum | [54] | |
| Kluyveromyces marxianus + sodium bicarbonate | Competition for nutrient and space. The salt stimulates K. marxianus growth and it inhibits fungal spore germination | P. digitatum | [55] | |
| Rhodosporidium paludigenum | Fruit resistance induction. Increase in ethylene production and expression of defensive genes | P. digitatum | [56] | |
| Pichia membranifaciens | Competition for nutrients or space | P. digitatum | [57] | |
| Metschnikowia pulcherrima, and Aureobasidium pullulans | Competition for nutrients and fruit resistance induction by influencing peroxidase and superoxide dismutase activities | P. digitatum P. italicum | [52] | |
| Candida stellimalicola | ‘Killer’ activity, production of chitinase, and inhibition of conidial germination | P. italicum | [44] | |
| Cryptococcus laurentii associated with cinnamic acid | Different influence of cinnamic acid on the antagonistic yeast and the pathogen, leading to synergistic effect | P. italicum | [58] | |
| Metschnikowia citriensis | Biofilm formation, adhesion to mycelia, and iron depletion | P. digitatum P. italicum | [53] | |
| Pseudozyma antarctica | Direct parasitism | P. digitatum P. italicum | [53] | |
| Rhodotorula minuta, Candida azyma, and Aureobasidium pullulans | ‘Killer’ activity and hydrolytic enzyme production | G. citri-aurantii | [11] | |
| Debaryomyces hansenii | Competition for space and nutrients | P. digitatum P. italicum | [32,59,60] | |
| Kazachstania exígua and Pichia fermentans | ‘Killer’ activity | P. digitatum P. italicum | [41] | |
| Bacillus subtilis | Water soluble antibiotics, proteins, enzymes, and VOC production | P. digitatum | [43,45] | |
| Bacteria | Bacillus amyloliquefaciens | Great amounts of antibiotics produced in vitro, however, still not effective for green mold control in vivo | P. digitatum | [46] |
| Lactobacillus plantarum | Metabolites 3-phenyllactic acid and benzeneacetic acid, 2-propenyl ester with antifungal activity | P. digitatum | [47,48] | |
| Streptomyces sp. | Metabolites with higher mass than 2000 and fungicidal effect | P. digitatum G. citri-aurantii | [49,50] | |
| Streptomyces violascens | Extracellular antifungal compounds that inhibits fungal spore germination and antibiosis | G. citri-aurantii | [61] |
| Plant/Fruit | Pathogen (s) | Extract/Method | Natural Products | Details | References |
|---|---|---|---|---|---|
| Chinese propolis | P. italicum | 1) Ethyl acetate (3 times); 2) chloroform; 3) ethanol and water; 4) methanol | Pinocembrin | Pinocembrin acts against P. italicum through inhibition on respiration and interference of energy homeostasis | [72] |
| Citrus aurantium | P. digitatum P. italicum | Hydrodistillation (peels, leaves, and flowers) | α-terpineol, terpinen-4-ol, linalool, and limonene | Essential oils (EOs) of flowers and leaves reduced the growth of pathogen, while EO of peels was inactive | [73] |
| Citrus eticulate Blanco | P. digitatum | - | Citral | Antifungal activity of citral was tested in vitro and in vivo and combined with the wax showed potential for control applications | [68] |
| Citrus fruits | P. italicum P. digitatum | Commercial product | Octanal | Octanal inhibits the fungal mycelial growth | [74] |
| Citrus fruits | P. italicum | Commercial product | Citral | Citral inhibits the mycelial growth of P. italicum causing disruption of cell membrane integrity | [75] |
| Citrus paradise Macf. (Grapefruit fruit) | P. digitatum | - | Chitosan and salicylic acid | Chitosan combined with salicylic acid had better treatment of green mold than these isolated compounds, without compromising the quality of fruit. | [26] |
| Citrus sinensis Osbeck | P. digitatum | Commercial product | Citronellal | Citronellal was able to inhibit spores germination and mycelial growth. Just as citral, the compound combined with wax reduced the incidence rate | [69] |
| Laminaceae spp. | P. digitatum P. italicum | - | Carvacrol and thymol | The mechanisms that have been proposed for these compounds are: 1) morphological deformation and deterioration of the conidia and hyphae; 2) hydroxyl group and systems with delocalized electrons has important role for antimicrobial effect | [69] |
| Peganum harmala L. (harmal seeds) | P. italicum | Ethanol | Harmine, harmaline, and tetrahydroharmine (THH) | Harmal extracts showed strong antifungal activity against P. italicum and its activity is related to alkaloids harmine, harmaline e THH | [76] |
| Peganum harmala L. | P. digitatum | Commercial product | Harmol, harmaline, harmalol, harmane, and norharmane | It was tested the antifungal activity of β-carbolines against P. digitatum and Botrytis cinerea. Harmol showed highest antifungal activity after 24 h. | [63] |
| Pimpinella anisum and Carum carvi | P. digitatum | Hydrodistillation (seeds) | trans-anethole, estragole (anise oil), cuminaldehyde, and perillaldehyde (black caraway) | EO were able in vitro of reduce the germination, the mycelial growth of pathogen and the incidence of disease symptoms | [77] |
| Populus × euramericana cv. ‘Neva’ (poplar buds) | P. italicum | Dichloromethane | Flavonoids of pinocembrin, chrysin, and galangin | Antifungal compounds from poplar buds active fraction, identified by HPLC–MS, had antifungal effect in the fungal hyphae analyzed by scanning electron microscopy and transmission electron microscopy images | [78] |
| Punica Granatum | P. digitatum | Ethanol/water (4:1) | Phenolic compounds with a prevalence of punicalagins | Pomegranate peel extract has a broad range of antifungal activity | [13] |
| Ramulus cinnamomi | P. digitatum P. italicum G. citri-aurantii | Ethyl acetate and n-buthanol | Cinnamic acid and cinnamaldehyde | Through 1H-NMR-based metabolomics it was identified the extracts related to antifungal activity of Ramulus cinnamomi after 4, 8, and 12 h. The antifungal mechanism of cinnamaldehyde it was also analyzed by 1H-NMR | [79] |
| Rosmarinus officinalis L. | P. digitatum | Hydrodistillation (for EO) and methanol | Flavonoids, polyphenols, and essential oils | EO act in the fungal cells by disrupting the membrane permeability and the osmotic balance | [80] |
| Salvia fruticosa Mill. | P. digitatum | Ethyl acetate | Carnosic acid, carnosol, and hispidulin | Compounds that have antifungal properties, according to its compositions, structures/activity, and literature | [81] |
| Sapium baccatum | P. digitatum | Commercial product | Tannic acid | In vitro antifungal activity to P. digitatum was verified between 400 and 1000 µg mL−1 of tannic acid inoculated in Ponkan fruit was sufficient to inhibit the mycelial growth of 45% to 100% | [82] |
| Solanum nigrum | P. digitatum | Aqueous extract (leaves) | Alkaloids, flavonoids, saponins, steroids, glycosides, terpenoids, and tannins | Bioactive compounds that has pharmacological prospects for development of drugs | [83] |
| Thymus species (T. leptobotris, T. riatarum, T. broussonnetii subsp. hannonis, and T. satureioides subsp. pseudomastichina) | P. digitatum P. italicum G. citri-aurantii | Hydrodistillation | Thymol, carvacrol, geraniol, eugenol, octanal, and citral | EO of four Thymus species showed antifungal activity. Through GC–MS, MIC, and previous studies determined the principal active compounds | [84] |
| Thymus leptobotris | P. digitatum P. italicum G. citri-aurantii | Methanol, chloroform | Thymol and carvacrol | The antifungal screening from EO obtained from 21 plants showed that the EO from Thymus leptobotris had the highest fungistatic effect. The active compounds were identified in previous studies. | [85] |
| Thymus vulgaris L. | P. italicum P. digitatum | - | Thymol | EO of thyme inhibited the mycelium growth (MIC 0.13 µL mL−1) and spore germination (MIC 0.50 µL mL−1) in vitro and in vivo | [86] |
| Withania somnifera + Acacia seyal | P. digitatum | Methanol/acetone/water—7:7:1, v/v (dried plant powder—1:20 w/v) | Insoluble and soluble phenolic compounds | Application of plants extract (W. somnifera and A. seyal) in the sick host, induced plant resistance through change of phenolic concentration (phenylpropanoid pathway) | [87] |
| Microorganism | Product | Targeted Pathogens | References |
|---|---|---|---|
| Candida oleophila | Aspire | Botrytis, Penicillium | [117] |
| Metschnikowia fructicola | Shemer | Botrytis, Penicillium, Rhizopus, Aspergillus | [124] |
| Pantoea agglomerans | Pantovital | Penicillium, Botrytis, Monilinia | [125] |
| Pseudomonas syringae | Biosave | Penicillium, Botrytis, Mucor | [126] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes Bazioli, J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.d.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080460
Moraes Bazioli J, Belinato JR, Costa JH, Akiyama DY, Pontes JGdM, Kupper KC, Augusto F, de Carvalho JE, Fill TP. Biological Control of Citrus Postharvest Phytopathogens. Toxins. 2019; 11(8):460. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080460
Chicago/Turabian StyleMoraes Bazioli, Jaqueline, João Raul Belinato, Jonas Henrique Costa, Daniel Yuri Akiyama, João Guilherme de Moraes Pontes, Katia Cristina Kupper, Fabio Augusto, João Ernesto de Carvalho, and Taícia Pacheco Fill. 2019. "Biological Control of Citrus Postharvest Phytopathogens" Toxins 11, no. 8: 460. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080460